Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum
- PMID: 30567719
- PMCID: PMC6314534
- DOI: 10.1084/jem.20181170
Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum
Abstract
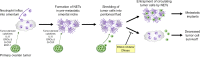
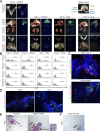
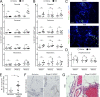
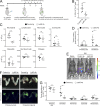
Ovarian cancer preferentially metastasizes to the omentum, a fatty tissue characterized by immune structures called milky spots, but the cellular dynamics that direct this tropism are unknown. Here, we identified that neutrophil influx into the omentum is a prerequisite premetastatic step in orthotopic ovarian cancer models. Ovarian tumor-derived inflammatory factors stimulated neutrophils to mobilize and extrude chromatin webs called neutrophil extracellular traps (NETs). NETs were detected in the omentum of ovarian tumor-bearing mice before metastasis and of women with early-stage ovarian cancer. NETs, in turn, bound ovarian cancer cells and promoted metastasis. Omental metastasis was decreased in mice with neutrophil-specific deficiency of peptidylarginine deiminase 4 (PAD4), an enzyme that is essential for NET formation. Blockade of NET formation using a PAD4 pharmacologic inhibitor also decreased omental colonization. Our findings implicate NET formation in rendering the premetastatic omental niche conducive for implantation of ovarian cancer cells and raise the possibility that blockade of NET formation prevents omental metastasis.
© 2018 Lee et al.
Figures
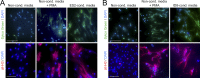
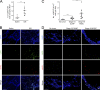
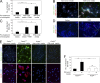


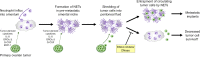
Comment in
-
Neutrophil: A mobile fertilizer.J Exp Med. 2019 Jan 7;216(1):4-6. doi: 10.1084/jem.20182059. Epub 2018 Dec 19. J Exp Med. 2019. PMID: 30567720 Free PMC article.
Similar articles
-
G-CSF induces neutrophil extracellular traps formation and promotes ovarian cancer peritoneal dissemination.J Leukoc Biol. 2024 Nov 4;116(5):1157-1168. doi: 10.1093/jleuko/qiae166. J Leukoc Biol. 2024. PMID: 39082070
-
Omental macrophages secrete chemokine ligands that promote ovarian cancer colonization of the omentum via CCR1.Commun Biol. 2020 Sep 22;3(1):524. doi: 10.1038/s42003-020-01246-z. Commun Biol. 2020. PMID: 32963283 Free PMC article.
-
In vitro metastatic colonization of human ovarian cancer cells to the omentum.Clin Exp Metastasis. 2010 Mar;27(3):185-96. doi: 10.1007/s10585-010-9317-0. Epub 2010 Mar 14. Clin Exp Metastasis. 2010. PMID: 20229256
-
Revisiting the Use of Normal Saline for Peritoneal Washing in Ovarian Cancer.Int J Mol Sci. 2023 Nov 17;24(22):16449. doi: 10.3390/ijms242216449. Int J Mol Sci. 2023. PMID: 38003636 Free PMC article. Review.
-
The Emerging Role of Neutrophil Extracellular Traps (NETs) in Tumor Progression and Metastasis.Front Immunol. 2020 Sep 16;11:1749. doi: 10.3389/fimmu.2020.01749. eCollection 2020. Front Immunol. 2020. PMID: 33042107 Free PMC article. Review.
Cited by
-
Neutrophil extracellular traps in tumor progression of gynecologic cancers.Front Immunol. 2024 Nov 1;15:1421889. doi: 10.3389/fimmu.2024.1421889. eCollection 2024. Front Immunol. 2024. PMID: 39555072 Free PMC article. Review.
-
Metformin may improve the outcome of patients with colorectal cancer and type 2 diabetes mellitus partly through effects on neutrophil extracellular traps.BJC Rep. 2023 Dec 12;1(1):20. doi: 10.1038/s44276-023-00022-w. BJC Rep. 2023. PMID: 39516686 Free PMC article.
-
Cancer-induced systemic pre-conditioning of distant organs: building a niche for metastatic cells.Nat Rev Cancer. 2024 Dec;24(12):829-849. doi: 10.1038/s41568-024-00752-0. Epub 2024 Oct 10. Nat Rev Cancer. 2024. PMID: 39390247 Review.
-
Proanthocyanidin Regulates NETosis and Inhibits the Growth and Proliferation of Liver Cancer Cells - In Vivo, In Vitro and In Silico Investigation.Cell Biochem Biophys. 2024 Oct 9. doi: 10.1007/s12013-024-01557-6. Online ahead of print. Cell Biochem Biophys. 2024. PMID: 39382828
-
Exploration of the link between COVID-19 and gastric cancer from the perspective of bioinformatics and systems biology.Front Med (Lausanne). 2024 Sep 20;11:1428973. doi: 10.3389/fmed.2024.1428973. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39371335 Free PMC article.
References
-
- Albrengues J., Shields M.A., Ng D., Park C.G., Ambrico A., Poindexter M.E., Upadhyay P., Uyeminami D.L., Pommier A., Küttner V., et al. . 2018. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 361:eaao4227 10.1126/science.aao4227 - DOI - PMC - PubMed
-
- Buscher K., Wang H., Zhang X., Striewski P., Wirth B., Saggu G., Lütke-Enking S., Mayadas T.N., Ley K., Sorokin L., and Song J.. 2016. Protection from septic peritonitis by rapid neutrophil recruitment through omental high endothelial venules. Nat. Commun. 7:10828 10.1038/ncomms10828 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

