Properties of single-channel and whole cell Cl- currents in guinea pig detrusor smooth muscle cells
- PMID: 30566392
- PMCID: PMC6580156
- DOI: 10.1152/ajpcell.00327.2018
Properties of single-channel and whole cell Cl- currents in guinea pig detrusor smooth muscle cells
Abstract
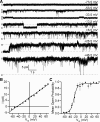
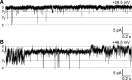
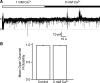
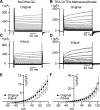
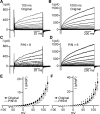
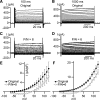
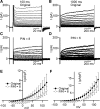
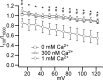
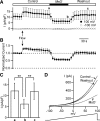
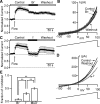
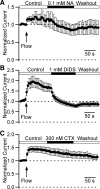
Multiple types of Cl- channels regulate smooth muscle excitability and contractility in vascular, gastrointestinal, and airway smooth muscle cells. However, little is known about Cl- channels in detrusor smooth muscle (DSM) cells. Here, we used inside-out single channel and whole cell patch-clamp recordings for detailed biophysical and pharmacological characterizations of Cl- channels in freshly isolated guinea pig DSM cells. The recorded single Cl- channels displayed unique gating with multiple subconductive states, a fully opened single-channel conductance of 164 pS, and a reversal potential of -41.5 mV, which is close to the ECl of -65 mV, confirming preferential permeability to Cl-. The Cl- channel demonstrated strong voltage dependence of activation (half-maximum of mean open probability, V0.5, ~-20 mV) and robust prolonged openings at depolarizing voltages. The channel displayed similar gating when exposed intracellularly to solutions containing Ca2+-free or 1 mM Ca2+. In whole cell patch-clamp recordings, macroscopic current demonstrated outward rectification, inhibitions by 4,4'-diisothiocyano-2,2'-stilbenedisulfonic acid (DIDS) and niflumic acid, and insensitivity to chlorotoxin. The outward current was reversibly reduced by 94% replacement of extracellular Cl- with I-, Br-, or methanesulfonate (MsO-), resulting in anionic permeability sequence: Cl->Br->I->MsO-. While intracellular Ca2+ levels (0, 300 nM, and 1 mM) did not affect the amplitude of Cl- current and outward rectification, high Ca2+ slowed voltage-step current activation at depolarizing voltages. In conclusion, our data reveal for the first time the presence of a Ca2+-independent DIDS and niflumic acid-sensitive, voltage-dependent Cl- channel in the plasma membrane of DSM cells. This channel may be a key regulator of DSM excitability.
Keywords: chloride; detrusor; ion channel; smooth muscle cell; urinary bladder.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
Similar articles
-
TRPM4 channel inhibitors 9-phenanthrol and glibenclamide differentially decrease guinea pig detrusor smooth muscle whole-cell cation currents and phasic contractions.Am J Physiol Cell Physiol. 2020 Feb 1;318(2):C406-C421. doi: 10.1152/ajpcell.00055.2019. Epub 2019 Dec 18. Am J Physiol Cell Physiol. 2020. PMID: 31851526 Free PMC article.
-
Urinary bladder smooth muscle ion channels: expression, function, and regulation in health and disease.Am J Physiol Renal Physiol. 2020 Aug 1;319(2):F257-F283. doi: 10.1152/ajprenal.00048.2020. Epub 2020 Jul 6. Am J Physiol Renal Physiol. 2020. PMID: 32628539 Free PMC article. Review.
-
Extracellular pH and intracellular phosphatidylinositol 4,5-bisphosphate control Cl- currents in guinea pig detrusor smooth muscle cells.Am J Physiol Cell Physiol. 2019 Dec 1;317(6):C1268-C1277. doi: 10.1152/ajpcell.00189.2019. Epub 2019 Oct 2. Am J Physiol Cell Physiol. 2019. PMID: 31577513 Free PMC article.
-
Testosterone decreases urinary bladder smooth muscle excitability via novel signaling mechanism involving direct activation of the BK channels.Am J Physiol Renal Physiol. 2016 Dec 1;311(6):F1253-F1259. doi: 10.1152/ajprenal.00238.2016. Epub 2016 Sep 7. Am J Physiol Renal Physiol. 2016. PMID: 27605581 Free PMC article.
-
Detrusor Smooth Muscle KV7 Channels: Emerging New Regulators of Urinary Bladder Function.Front Physiol. 2020 Sep 16;11:1004. doi: 10.3389/fphys.2020.01004. eCollection 2020. Front Physiol. 2020. PMID: 33041840 Free PMC article. Review.
Cited by
-
TRPM4 channel inhibitors 9-phenanthrol and glibenclamide differentially decrease guinea pig detrusor smooth muscle whole-cell cation currents and phasic contractions.Am J Physiol Cell Physiol. 2020 Feb 1;318(2):C406-C421. doi: 10.1152/ajpcell.00055.2019. Epub 2019 Dec 18. Am J Physiol Cell Physiol. 2020. PMID: 31851526 Free PMC article.
-
Preparation and Utilization of Freshly Isolated Human Detrusor Smooth Muscle Cells for Characterization of 9-Phenanthrol-Sensitive Cation Currents.J Vis Exp. 2020 Jan 31;(155):10.3791/59884. doi: 10.3791/59884. J Vis Exp. 2020. PMID: 32065126 Free PMC article.
-
Urinary bladder smooth muscle ion channels: expression, function, and regulation in health and disease.Am J Physiol Renal Physiol. 2020 Aug 1;319(2):F257-F283. doi: 10.1152/ajprenal.00048.2020. Epub 2020 Jul 6. Am J Physiol Renal Physiol. 2020. PMID: 32628539 Free PMC article. Review.
-
Chloride channels with ClC-1-like properties differentially regulate the excitability of dopamine receptor D1- and D2-expressing striatal medium spiny neurons.Am J Physiol Cell Physiol. 2022 Mar 1;322(3):C395-C409. doi: 10.1152/ajpcell.00397.2021. Epub 2022 Jan 26. Am J Physiol Cell Physiol. 2022. PMID: 35080921 Free PMC article.
-
Extracellular pH and intracellular phosphatidylinositol 4,5-bisphosphate control Cl- currents in guinea pig detrusor smooth muscle cells.Am J Physiol Cell Physiol. 2019 Dec 1;317(6):C1268-C1277. doi: 10.1152/ajpcell.00189.2019. Epub 2019 Oct 2. Am J Physiol Cell Physiol. 2019. PMID: 31577513 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

