Monitoring HIV DNA and cellular activation markers in HIV-infected humanized mice under cART
- PMID: 30558630
- PMCID: PMC6296118
- DOI: 10.1186/s12985-018-1101-9
Monitoring HIV DNA and cellular activation markers in HIV-infected humanized mice under cART
Abstract
Background: The major obstacle to cure of HIV type-1 infection is the presence of the HIV reservoir, hidden from the immune system and insensitive to combined antiretroviral therapy (cART). Eradication approaches have been hindered by the difficulty for accurately monitoring its size in vivo, especially in the lymphoid organs. Humanized mouse models are a valuable tool for systematically assess the efficacy of therapeutic interventions in reducing the HIV reservoir. Nonetheless, persistence of the HIV reservoir over time, in the presence of cART, has yet to be analyzed in this in vivo model.
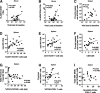
Findings: We found that the proviral DNA as well as the total DNA were very stable in the spleen and mesenteric lymph node irrespective of the length of cART. Notably, the amount of proviral DNA was very similar in the spleen and lymph node. Furthermore, we observed a correlation between the percentage of splenic human CD4+ T-cells with total HIV DNA, between the number of human CD38 + CD8+ T-cells in the spleen with the amount of integrated HIV DNA, and eventually between the hCD4/hCD8 ratio in the spleen with integrated as well as total HIV DNA implying that the CD8+ T cells influence the size of the HIV reservoir.
Conclusions: Here, we demonstrated the stability of this reservoir in humanized mice irrespective of the length of cART, confirming the relevancy of this model for HIV latency eradication investigations. Notably, we also found correlates between the frequency of CD4+ T-cells, their activation status and viral parameters, which were analogous to the ones in HIV-infected patients. Thus, hu-mice represent a very valuable HIV latency model.
Keywords: Alu-PCR; HIV reservoir size; HIV-1; Humanized mice; cART.
Conflict of interest statement
Ethics approval and consent to participate
All humanized mouse experiments as well as the procurement of human cord blood were approved by ethical committees of the University of Zurich and the Federal Veterinary Departments. The experiments were following the local guidelines (TschV, Zurich) and the Swiss animal protection law (TschG). Human cord blood was obtained with informed written consent of the parents and processed anonymously.
Consent for publication
All authors consent for publication.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Genetic Diversity, Compartmentalization, and Age of HIV Proviruses Persisting in CD4+ T Cell Subsets during Long-Term Combination Antiretroviral Therapy.J Virol. 2020 Feb 14;94(5):e01786-19. doi: 10.1128/JVI.01786-19. Print 2020 Feb 14. J Virol. 2020. PMID: 31776273 Free PMC article.
-
Peripheral T Follicular Helper Cells Are the Major HIV Reservoir within Central Memory CD4 T Cells in Peripheral Blood from Chronically HIV-Infected Individuals on Combination Antiretroviral Therapy.J Virol. 2015 Dec 16;90(6):2718-28. doi: 10.1128/JVI.02883-15. J Virol. 2015. PMID: 26676775 Free PMC article. Clinical Trial.
-
The role of integration and clonal expansion in HIV infection: live long and prosper.Retrovirology. 2018 Oct 23;15(1):71. doi: 10.1186/s12977-018-0448-8. Retrovirology. 2018. PMID: 30352600 Free PMC article. Review.
-
Continued Decay of HIV Proviral DNA Upon Vaccination With HIV-1 Tat of Subjects on Long-Term ART: An 8-Year Follow-Up Study.Front Immunol. 2019 Feb 13;10:233. doi: 10.3389/fimmu.2019.00233. eCollection 2019. Front Immunol. 2019. PMID: 30815001 Free PMC article. Clinical Trial.
-
HIV-specific CD8⁺ T cells and HIV eradication.J Clin Invest. 2016 Feb;126(2):455-63. doi: 10.1172/JCI80566. Epub 2016 Jan 5. J Clin Invest. 2016. PMID: 26731469 Free PMC article. Review.
Cited by
-
Validation of a UHPLC-MS/MS Method to Quantify Twelve Antiretroviral Drugs within Peripheral Blood Mononuclear Cells from People Living with HIV.Pharmaceuticals (Basel). 2020 Dec 25;14(1):12. doi: 10.3390/ph14010012. Pharmaceuticals (Basel). 2020. PMID: 33375547 Free PMC article.
-
HIV Replication in Humanized IL-3/GM-CSF-Transgenic NOG Mice.Pathogens. 2019 Mar 12;8(1):33. doi: 10.3390/pathogens8010033. Pathogens. 2019. PMID: 30871027 Free PMC article.
-
Advances in Humanized Mouse Models to Improve Understanding of HIV-1 Pathogenesis and Immune Responses.Front Immunol. 2021 Mar 5;11:617516. doi: 10.3389/fimmu.2020.617516. eCollection 2020. Front Immunol. 2021. PMID: 33746940 Free PMC article. Review.
-
Recent Developments in NSG and NRG Humanized Mouse Models for Their Use in Viral and Immune Research.Viruses. 2023 Feb 9;15(2):478. doi: 10.3390/v15020478. Viruses. 2023. PMID: 36851692 Free PMC article. Review.
-
HIV-1 DNA levels in peripheral blood mononuclear cells of patients with HIV-related non-Hodgkin's lymphoma.Chin Med J (Engl). 2023 Nov 20;136(22):2741-2743. doi: 10.1097/CM9.0000000000002897. Epub 2023 Nov 3. Chin Med J (Engl). 2023. PMID: 37926994 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

