CD8+ T Cells Responding to the Middle East Respiratory Syndrome Coronavirus Nucleocapsid Protein Delivered by Vaccinia Virus MVA in Mice
- PMID: 30558354
- PMCID: PMC6316859
- DOI: 10.3390/v10120718
CD8+ T Cells Responding to the Middle East Respiratory Syndrome Coronavirus Nucleocapsid Protein Delivered by Vaccinia Virus MVA in Mice
Abstract
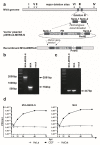
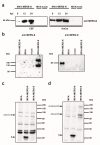
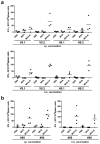
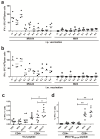
Middle East respiratory syndrome coronavirus (MERS-CoV), a novel infectious agent causing severe respiratory disease and death in humans, was first described in 2012. Antibodies directed against the MERS-CoV spike (S) protein are thought to play a major role in controlling MERS-CoV infection and in mediating vaccine-induced protective immunity. In contrast, relatively little is known about the role of T cell responses and the antigenic targets of MERS-CoV that are recognized by CD8+ T cells. In this study, the highly conserved MERS-CoV nucleocapsid (N) protein served as a target immunogen to elicit MERS-CoV-specific cellular immune responses. Modified Vaccinia virus Ankara (MVA), a safety-tested strain of vaccinia virus for preclinical and clinical vaccine research, was used for generating MVA-MERS-N expressing recombinant N protein. Overlapping peptides spanning the whole MERS-CoV N polypeptide were used to identify major histocompatibility complex class I/II-restricted T cell responses in BALB/c mice immunized with MVA-MERS-N. We have identified a H2-d restricted decamer peptide epitope in the MERS-N protein with CD8+ T cell antigenicity. The identification of this epitope, and the availability of the MVA-MERS-N candidate vaccine, will help to evaluate MERS-N-specific immune responses and the potential immune correlates of vaccine-mediated protection in the appropriate murine models of MERS-CoV infection.
Keywords: MERS-CoV; MERS-CoV nucleocapsid protein; MVA vaccine; murine CD8+ T cell epitope.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Protective Efficacy of Recombinant Modified Vaccinia Virus Ankara Delivering Middle East Respiratory Syndrome Coronavirus Spike Glycoprotein.J Virol. 2015 Aug;89(16):8651-6. doi: 10.1128/JVI.00614-15. Epub 2015 May 27. J Virol. 2015. PMID: 26018172 Free PMC article.
-
Epitope-Based Vaccine Target Screening against Highly Pathogenic MERS-CoV: An In Silico Approach Applied to Emerging Infectious Diseases.PLoS One. 2015 Dec 7;10(12):e0144475. doi: 10.1371/journal.pone.0144475. eCollection 2015. PLoS One. 2015. PMID: 26641892 Free PMC article.
-
A Highly Immunogenic and Protective Middle East Respiratory Syndrome Coronavirus Vaccine Based on a Recombinant Measles Virus Vaccine Platform.J Virol. 2015 Nov;89(22):11654-67. doi: 10.1128/JVI.01815-15. Epub 2015 Sep 9. J Virol. 2015. PMID: 26355094 Free PMC article.
-
Middle East Respiratory Syndrome Coronavirus (MERS-CoV): Infection, Immunological Response, and Vaccine Development.J Immunol Res. 2019 Apr 7;2019:6491738. doi: 10.1155/2019/6491738. eCollection 2019. J Immunol Res. 2019. PMID: 31089478 Free PMC article. Review.
-
Progress of Middle East respiratory syndrome coronavirus vaccines: a patent review.Expert Opin Ther Pat. 2017 Jun;27(6):721-731. doi: 10.1080/13543776.2017.1281248. Epub 2017 Jan 25. Expert Opin Ther Pat. 2017. PMID: 28121202 Review.
Cited by
-
Generation of SARS-CoV-2 Mouse Model by Transient Expression of the Human ACE2 Gene Mediated by Intranasal Administration of AAV-hACE2.Mol Biol. 2022;56(5):705-712. doi: 10.1134/S0026893322050065. Epub 2022 Oct 5. Mol Biol. 2022. PMID: 36217340 Free PMC article.
-
Immune Response to COVID-19: Can We Benefit from the SARS-CoV and MERS-CoV Pandemic Experience?Pathogens. 2020 Sep 9;9(9):739. doi: 10.3390/pathogens9090739. Pathogens. 2020. PMID: 32916812 Free PMC article. Review.
-
Recent Advances in the Vaccine Development Against Middle East Respiratory Syndrome-Coronavirus.Front Microbiol. 2019 Aug 2;10:1781. doi: 10.3389/fmicb.2019.01781. eCollection 2019. Front Microbiol. 2019. PMID: 31428074 Free PMC article. Review.
-
Immunoinformatic Analysis of SARS-CoV-2 Nucleocapsid Protein and Identification of COVID-19 Vaccine Targets.Front Immunol. 2020 Oct 28;11:587615. doi: 10.3389/fimmu.2020.587615. eCollection 2020. Front Immunol. 2020. PMID: 33193414 Free PMC article.
-
Single MVA-SARS-2-ST/N Vaccination Rapidly Protects K18-hACE2 Mice against a Lethal SARS-CoV-2 Challenge Infection.Viruses. 2024 Mar 8;16(3):417. doi: 10.3390/v16030417. Viruses. 2024. PMID: 38543782 Free PMC article.
References
-
- WHO Middle East respiratory Syndrome Coronavirus (MERS-CoV) [(accessed on 22 November 2018)]; Available online: http://www.who.int/emergencies/mers-cov/en/
-
- Alraddadi B.M., Watson J.T., Almarashi A., Abedi G.R., Turkistani A., Sadran M., Housa A., Almazroa M.A., Alraihan N., Banjar A., et al. Risk Factors for Primary Middle East Respiratory Syndrome Coronavirus Illness in Humans, Saudi Arabia, 2014. Emerg. Infect. Dis. 2016;22:49–55. doi: 10.3201/eid2201.151340. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

