CD32 Ligation Promotes the Activation of CD4+ T Cells
- PMID: 30555482
- PMCID: PMC6284025
- DOI: 10.3389/fimmu.2018.02814
CD32 Ligation Promotes the Activation of CD4+ T Cells
Abstract
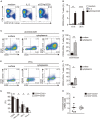
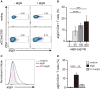
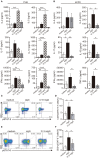
Low affinity receptors for the Fc portion of IgG (FcγRs) represent a critical link between innate and adaptive immunity. Immune complexes (ICs) are the natural ligands for low affinity FcγRs, and high levels of ICs are usually detected in both, chronic viral infections and autoimmune diseases. The expression and function of FcγRs in myeloid cells, NK cells and B cells have been well characterized. By contrast, there are controversial reports about the expression and function of FcγRs in T cells. Here, we demonstrated that ~2% of resting CD4+ T cells express cell surface FcγRII (CD32). Analysis of CD32 expression in permeabilized cells revealed an increased proportion of CD4+CD32+ T cells (~9%), indicating that CD4+ T cells store a CD32 cytoplasmic pool. Activation of CD4+ T cells markedly increased the expression of CD32 either at the cell surface or intracellularly. Analysis of CD32 mRNA transcripts in activated CD4+ T cells revealed the presence of both, the stimulatory FcγRIIa (CD32a) and the inhibitory FcγRIIb (CD32b) isoforms of CD32, being the CD32a:CD32b mRNA ratio ~5:1. Consistent with this finding, we found not only that CD4+ T cells bind aggregated IgG, used as an IC model, but also that CD32 ligation by specific mAb induced a strong calcium transient in CD4+ T cells. Moreover, we found that pretreatment of CD4+ T cells with immobilized IgG as well as cross-linking of CD32 by specific antibodies increased both, the proliferative response of CD4+ T cells and the release of a wide pattern of cytokines (IL-2, IL-5, IL-10, IL-17, IFN-γ, and TNF-α) triggered by either PHA or anti-CD3 mAb. Collectively, our results indicate that ligation of CD32 promotes the activation of CD4+ T cells. These findings suggest that ICs might contribute to the perpetuation of chronic inflammatory responses by virtue of its ability to directly interact with CD4+ T cells through CD32a, promoting the activation of T cells into different inflammatory profiles.
Keywords: FcγR; IgG; T cells; activation; cytokines; proliferation.
Figures


Similar articles
-
Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation.J Immunol. 1993 Jun 1;150(11):4754-65. J Immunol. 1993. PMID: 7684412
-
Anti-FcγRIIB (CD32) Antibodies Differentially Modulate Murine FVIII-Specific Recall Response in vitro.Scand J Immunol. 2017 Aug;86(2):91-99. doi: 10.1111/sji.12573. Scand J Immunol. 2017. PMID: 28561280
-
Monoclonal antibodies capable of discriminating the human inhibitory Fcgamma-receptor IIB (CD32B) from the activating Fcgamma-receptor IIA (CD32A): biochemical, biological and functional characterization.Immunology. 2007 Jul;121(3):392-404. doi: 10.1111/j.1365-2567.2007.02588.x. Epub 2007 Mar 26. Immunology. 2007. PMID: 17386079 Free PMC article.
-
The Human FcγRII (CD32) Family of Leukocyte FcR in Health and Disease.Front Immunol. 2019 Mar 19;10:464. doi: 10.3389/fimmu.2019.00464. eCollection 2019. Front Immunol. 2019. PMID: 30941127 Free PMC article. Review.
-
Functional regulation of human neutrophil Fc gamma receptors.Immunol Res. 2004;29(1-3):219-30. doi: 10.1385/IR:29:1-3:219. Immunol Res. 2004. PMID: 15181284 Review.
Cited by
-
sFgl2 gene-modified MSCs regulate the differentiation of CD4+ T cells in the treatment of autoimmune hepatitis.Stem Cell Res Ther. 2023 Nov 3;14(1):316. doi: 10.1186/s13287-023-03550-x. Stem Cell Res Ther. 2023. PMID: 37924141 Free PMC article.
-
Quantitative Proteomic Analysis Reveals Functional Alterations of the Peripheral Immune System in Colorectal Cancer.Mol Cell Proteomics. 2024 Jun;23(6):100784. doi: 10.1016/j.mcpro.2024.100784. Epub 2024 May 11. Mol Cell Proteomics. 2024. PMID: 38735538 Free PMC article.
-
The Quest for Cellular Markers of HIV Reservoirs: Any Color You Like.Front Immunol. 2019 Sep 20;10:2251. doi: 10.3389/fimmu.2019.02251. eCollection 2019. Front Immunol. 2019. PMID: 31616425 Free PMC article. Review.
-
Multi-omics comprehensive analyses of programmed cell death patterns to regulate the immune characteristics of head and neck squamous cell carcinoma.Transl Oncol. 2024 Mar;41:101862. doi: 10.1016/j.tranon.2023.101862. Epub 2024 Jan 18. Transl Oncol. 2024. PMID: 38237211 Free PMC article.
-
pH Low Insertion Peptide-Modified Programmed Cell Death-Ligand 1 Potently Suppresses T-Cell Activation Under Acidic Condition.Front Immunol. 2021 Dec 23;12:794226. doi: 10.3389/fimmu.2021.794226. eCollection 2021. Front Immunol. 2021. PMID: 35003115 Free PMC article.
References
-
- Amigorena S, Bonnerot C, Drake JR, Choquet D, Hunziker W, Guillet JG, et al. . Cytoplasmic domain heterogeneity and functions of IgG Fc receptors in B lymphocytes. Science (1992) 256:1808–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

