Protective Effects and Target Network Analysis of Ginsenoside Rg1 in Cerebral Ischemia and Reperfusion Injury: A Comprehensive Overview of Experimental Studies
- PMID: 30545139
- PMCID: PMC6316103
- DOI: 10.3390/cells7120270
Protective Effects and Target Network Analysis of Ginsenoside Rg1 in Cerebral Ischemia and Reperfusion Injury: A Comprehensive Overview of Experimental Studies
Abstract
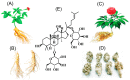
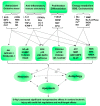
Cerebral ischemia-reperfusion is a complicated pathological process. The injury and cascade reactions caused by cerebral ischemia and reperfusion are characterized by high mortality, high recurrence, and high disability. However, only a limited number of antithrombotic drugs, such as recombinant tissue plasminogen activator (r-TPA), aspirin, and heparin, are currently available for ischemic stroke, and its safety concerns is inevitable which associated with reperfusion injury and hemorrhage. Therefore, it is necessary to further explore and examine some potential neuroprotective agents with treatment for cerebral ischemia and reperfusion injury to reduce safety concerns caused by antithrombotic drugs in ischemic stroke. Ginseng Rg1 (G-Rg1) is a saponin composed of natural active ingredients and derived from the roots or stems of Panax notoginseng and ginseng in traditional Chinese medicine. Its pharmacological effects exert remarkable neurotrophic and neuroprotective effects in the central nervous system. To explore and summarize the protective effects and mechanisms of ginsenoside Rg1 against cerebral ischemia and reperfusion injury, we conducted this review, in which we searched the PubMed database to obtain and organize studies concerning the pharmacological effects and mechanisms of ginsenoside Rg1 against cerebral ischemia and reperfusion injury. This study provides a valuable reference and clues for the development of new agents to combat ischemic stroke. Our summarized review and analysis show that the pharmacological effects of and mechanisms underlying ginsenoside Rg1 activity against cerebral ischemia and reperfusion injury mainly involve 4 sets of mechanisms: anti-oxidant activity and associated apoptosis via the Akt, Nrf2/HO-1, PPARγ/HO-1, extracellular regulated protein kinases (ERK), p38, and c-Jun N-terminal kinase (JNK) pathways (or mitochondrial apoptosis pathway) and the caspase-3/ROCK1/MLC pathway; anti-inflammatory and immune stimulatory-related activities that involve apoptosis or necrosis via MAPK pathways (the JNK1/2 + ERK1/2 and PPARγ/HO-1 pathways), endoplasmic reticulum stress (ERS), high mobility group protein1 (HMGB1)-induced TLR2/4/9 and receptor for advanced glycation end products (RAGE) pathways, and the activation of NF-κB; neurological cell cycle, proliferation, differentiation, and regeneration via the MAPK pathways (JNK1/2 + ERK1/2, PI3K-Akt/mTOR, PKB/Akt and HIF-1α/VEGF pathways); and energy metabolism and the regulation of cellular ATP levels, the blood-brain barrier and other effects via N-methyl-D-aspartic acid (NMDA) receptors, ERS, and AMP/AMPK-GLUT pathways. Collectively, these mechanisms result in significant neuroprotective effects against cerebral ischemic injury. These findings will be valuable in that they should further promote the development of candidate drugs and provide more information to support the application of previous findings in stroke clinical trials.
Keywords: anti-inflammatory; anti-oxidant; cerebral ischemia and reperfusion injury; differentiation; energy metabolism; ginsenoside Rg1; ischemia stroke; proliferation; review.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Effects of the Combination of the Main Active Components of Astragalus and Panax notoginseng on Inflammation and Apoptosis of Nerve Cell after Cerebral Ischemia-Reperfusion.Am J Chin Med. 2015;43(7):1419-38. doi: 10.1142/S0192415X15500809. Epub 2015 Oct 18. Am J Chin Med. 2015. PMID: 26477799
-
[Effects of ginsenoside Rg1 on the expressions of p-eRK1/2 and p-JNK in local cerebral ischemia/reperfusion injury rats].Zhongguo Zhong Xi Yi Jie He Za Zhi. 2013 Feb;33(2):229-34. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2013. PMID: 23646480 Chinese.
-
Ginsenoside Rg1 suppressed inflammation and neuron apoptosis by activating PPARγ/HO-1 in hippocampus in rat model of cerebral ischemia-reperfusion injury.Int J Clin Exp Pathol. 2015 Mar 1;8(3):2484-94. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26045754 Free PMC article.
-
Protective Effects and Network Analysis of Ginsenoside Rb1 Against Cerebral Ischemia Injury: A Pharmacological Review.Front Pharmacol. 2021 Jul 2;12:604811. doi: 10.3389/fphar.2021.604811. eCollection 2021. Front Pharmacol. 2021. PMID: 34276353 Free PMC article. Review.
-
[Advances in pharmacological studies of Panax notoginseng saponins on brain ischemia-reperfusion injury].Yao Xue Xue Bao. 2016 Jul;51(7):1039-46. Yao Xue Xue Bao. 2016. PMID: 29896950 Review. Chinese.
Cited by
-
Selective brain hypothermia-induced neuroprotection against focal cerebral ischemia/reperfusion injury is associated with Fis1 inhibition.Neural Regen Res. 2020 May;15(5):903-911. doi: 10.4103/1673-5374.268973. Neural Regen Res. 2020. PMID: 31719256 Free PMC article.
-
Ginsenoside Rg1 ameliorates apoptosis, senescence and oxidative stress in ox-LDL-induced vascular endothelial cells via the AMPK/SIRT3/p53 signaling pathway.Exp Ther Med. 2022 Jun 30;24(3):545. doi: 10.3892/etm.2022.11482. eCollection 2022 Sep. Exp Ther Med. 2022. PMID: 35978936 Free PMC article.
-
Panax notoginseng: derived exosome-like nanoparticles attenuate ischemia reperfusion injury via altering microglia polarization.J Nanobiotechnology. 2023 Nov 10;21(1):416. doi: 10.1186/s12951-023-02161-1. J Nanobiotechnology. 2023. PMID: 37946257 Free PMC article.
-
Potential Role of Natural Antioxidants in Countering Reperfusion Injury in Acute Myocardial Infarction and Ischemic Stroke.Antioxidants (Basel). 2023 Sep 13;12(9):1760. doi: 10.3390/antiox12091760. Antioxidants (Basel). 2023. PMID: 37760064 Free PMC article. Review.
-
p53 Inhibition Protects against Neuronal Ischemia/Reperfusion Injury by the p53/PRAS40/mTOR Pathway.Oxid Med Cell Longev. 2021 Dec 3;2021:4729465. doi: 10.1155/2021/4729465. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34900085 Free PMC article.
References
-
- Tuttolomondo A., Di S.R., Di R.D., Pedone C., La P.S., Pinto A., Licata G. Effects of clinical and laboratory variables and of pretreatment with cardiovascular drugs in acute ischaemic stroke: A retrospective chart review from the GIFA study. Int. J. Cardiol. 2011;151:318–322. doi: 10.1016/j.ijcard.2010.06.005. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

