Rapamycin, proliferation and geroconversion to senescence
- PMID: 30541374
- PMCID: PMC6343718
- DOI: 10.1080/15384101.2018.1554781
Rapamycin, proliferation and geroconversion to senescence
Abstract
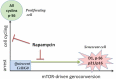
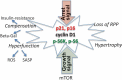
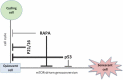
Rapamycin inhibits cell proliferation, yet preserves (re)-proliferative potential (RPP). RPP is a potential of quiescent cells that is lost in senescent cells. mTOR drives conversion from quiescence to senescence (geroconversion). By suppressing geroconversion, rapamycin preserves RPP. Geroconversion is characterized by proliferation-like levels of phospho-S6K/S6/4E-BP1 in nonproliferating cells arrested by p16 and/or p21. mTOR-driven geroconversion is associated with cellular hyperfunction, which in turn leads to organismal aging manifested by age-related diseases.
Keywords: Aging; SASP; cancer; gero-suppressants; rapalogs; senolytics.
Figures


Similar articles
-
CDK4/6-inhibiting drug substitutes for p21 and p16 in senescence: duration of cell cycle arrest and MTOR activity determine geroconversion.Cell Cycle. 2013 Sep 15;12(18):3063-9. doi: 10.4161/cc.26130. Epub 2013 Aug 22. Cell Cycle. 2013. PMID: 23974099 Free PMC article.
-
Dual mTORC1/C2 inhibitors suppress cellular geroconversion (a senescence program).Oncotarget. 2015 Sep 15;6(27):23238-48. doi: 10.18632/oncotarget.4836. Oncotarget. 2015. PMID: 26177051 Free PMC article.
-
MEK drives cyclin D1 hyperelevation during geroconversion.Cell Death Differ. 2013 Sep;20(9):1241-9. doi: 10.1038/cdd.2013.86. Epub 2013 Jul 12. Cell Death Differ. 2013. PMID: 23852369 Free PMC article.
-
Cell senescence, rapamycin and hyperfunction theory of aging.Cell Cycle. 2022 Jul;21(14):1456-1467. doi: 10.1080/15384101.2022.2054636. Epub 2022 Mar 31. Cell Cycle. 2022. PMID: 35358003 Free PMC article. Review.
-
Rapamycin and the inhibition of the secretory phenotype.Exp Gerontol. 2017 Aug;94:89-92. doi: 10.1016/j.exger.2017.01.026. Epub 2017 Feb 4. Exp Gerontol. 2017. PMID: 28167236 Review.
Cited by
-
Senescence-like phenotype in post-mitotic cells of mice entering middle age.Aging (Albany NY). 2020 Aug 5;12(14):13979-13990. doi: 10.18632/aging.103637. Aging (Albany NY). 2020. PMID: 32634782 Free PMC article.
-
PI3K/AKT/mTOR Signaling Regulates the Virus/Host Cell Crosstalk in HPV-Positive Cervical Cancer Cells.Int J Mol Sci. 2019 May 3;20(9):2188. doi: 10.3390/ijms20092188. Int J Mol Sci. 2019. PMID: 31058807 Free PMC article. Review.
-
Hydroxyurea Induces Bone Marrow Mesenchymal Stromal Cells Senescence and Modifies Cell Functionality In Vitro.J Pers Med. 2021 Oct 20;11(11):1048. doi: 10.3390/jpm11111048. J Pers Med. 2021. PMID: 34834400 Free PMC article.
-
The hyperfunction theory of aging: three common misconceptions.Oncoscience. 2021 Sep 17;8:103-107. doi: 10.18632/oncoscience.545. eCollection 2021. Oncoscience. 2021. PMID: 34549076 Free PMC article. No abstract available.
-
Obesity and hyperinsulinemia drive adipocytes to activate a cell cycle program and senesce.Nat Med. 2021 Nov;27(11):1941-1953. doi: 10.1038/s41591-021-01501-8. Epub 2021 Oct 4. Nat Med. 2021. PMID: 34608330
References
-
- Demidenko ZN, Blagosklonny MV.. Growth stimulation leads to cellular senescence when the cell cycle is blocked. Cell Cycle. 2008;7:3355–3361. - PubMed
-
- Shay JW, Pereira-Smith OM, Wright WE. A role for both RB and p53 in the regulation of human cellular senescence. Exp Cell Res. 1991;196(1):33–39. - PubMed
-
- Sage J, Miller AL, Perez-Mancera PA, et al. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature. 2003;424(6945):223–228. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
