l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans
- PMID: 30530985
- PMCID: PMC6307959
- DOI: 10.1172/JCI94601
l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans
Abstract
Background: l-Carnitine, an abundant nutrient in red meat, accelerates atherosclerosis in mice via gut microbiota-dependent formation of trimethylamine (TMA) and trimethylamine N-oxide (TMAO) via a multistep pathway involving an atherogenic intermediate, γ-butyrobetaine (γBB). The contribution of γBB in gut microbiota-dependent l-carnitine metabolism in humans is unknown.
Methods: Omnivores and vegans/vegetarians ingested deuterium-labeled l-carnitine (d3-l-carnitine) or γBB (d9-γBB), and both plasma metabolites and fecal polymicrobial transformations were examined at baseline, following oral antibiotics, or following chronic (≥2 months) l-carnitine supplementation. Human fecal commensals capable of performing each step of the l-carnitine→γBB→TMA transformation were identified.
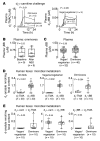
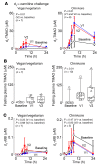
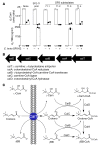
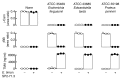
Results: Studies with oral d3-l-carnitine or d9-γBB before versus after antibiotic exposure revealed gut microbiota contribution to the initial 2 steps in a metaorganismal l-carnitine→γBB→TMA→TMAO pathway in subjects. Moreover, a striking increase in d3-TMAO generation was observed in omnivores over vegans/vegetarians (>20-fold; P = 0.001) following oral d3-l-carnitine ingestion, whereas fasting endogenous plasma l-carnitine and γBB levels were similar in vegans/vegetarians (n = 32) versus omnivores (n = 40). Fecal metabolic transformation studies, and oral isotope tracer studies before versus after chronic l-carnitine supplementation, revealed that omnivores and vegans/vegetarians alike rapidly converted carnitine to γBB, whereas the second gut microbial transformation, γBB→TMA, was diet inducible (l-carnitine, omnivorous). Extensive anaerobic subculturing of human feces identified no single commensal capable of l-carnitine→TMA transformation, multiple community members that converted l-carnitine to γBB, and only 1 Clostridiales bacterium, Emergencia timonensis, that converted γBB to TMA. In coculture, E. timonensis promoted the complete l-carnitine→TMA transformation.
Conclusion: In humans, dietary l-carnitine is converted into the atherosclerosis- and thrombosis-promoting metabolite TMAO via 2 sequential gut microbiota-dependent transformations: (a) initial rapid generation of the atherogenic intermediate γBB, followed by (b) transformation into TMA via low-abundance microbiota in omnivores, and to a markedly lower extent, in vegans/vegetarians. Gut microbiota γBB→TMA/TMAO transformation is induced by omnivorous dietary patterns and chronic l-carnitine exposure.
Trial registration: ClinicalTrials.gov NCT01731236.
Funding: NIH and Office of Dietary Supplements grants HL103866, HL126827, and DK106000, and the Leducq Foundation.
Keywords: Atherosclerosis; Cardiology; Cardiovascular disease; Vascular Biology.
Conflict of interest statement
Figures





Similar articles
-
The microbial gbu gene cluster links cardiovascular disease risk associated with red meat consumption to microbiota L-carnitine catabolism.Nat Microbiol. 2022 Jan;7(1):73-86. doi: 10.1038/s41564-021-01010-x. Epub 2021 Dec 23. Nat Microbiol. 2022. PMID: 34949826 Free PMC article.
-
γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO.Cell Metab. 2014 Nov 4;20(5):799-812. doi: 10.1016/j.cmet.2014.10.006. Epub 2014 Nov 4. Cell Metab. 2014. PMID: 25440057 Free PMC article.
-
Elucidation of an anaerobic pathway for metabolism of l-carnitine-derived γ-butyrobetaine to trimethylamine in human gut bacteria.Proc Natl Acad Sci U S A. 2021 Aug 10;118(32):e2101498118. doi: 10.1073/pnas.2101498118. Proc Natl Acad Sci U S A. 2021. PMID: 34362844 Free PMC article.
-
Metaorganismal nutrient metabolism as a basis of cardiovascular disease.Curr Opin Lipidol. 2014 Feb;25(1):48-53. doi: 10.1097/MOL.0000000000000036. Curr Opin Lipidol. 2014. PMID: 24362355 Free PMC article. Review.
-
Trimethylamine/Trimethylamine-N-Oxide as a Key Between Diet and Cardiovascular Diseases.Cardiovasc Toxicol. 2021 Aug;21(8):593-604. doi: 10.1007/s12012-021-09656-z. Epub 2021 May 18. Cardiovasc Toxicol. 2021. PMID: 34003426 Review.
Cited by
-
The Potential Use of Metabolic Cofactors in Treatment of NAFLD.Nutrients. 2019 Jul 12;11(7):1578. doi: 10.3390/nu11071578. Nutrients. 2019. PMID: 31336926 Free PMC article. Review.
-
PI3K/AKT/SERBP-1 pathway regulates Alisma orientalis beverage treatment of atherosclerosis in APOE-/- high-fat diet mice.Pharm Biol. 2023 Dec;61(1):473-487. doi: 10.1080/13880209.2023.2168020. Pharm Biol. 2023. PMID: 36825364 Free PMC article.
-
Immune mechanism of gut microbiota and its metabolites in the occurrence and development of cardiovascular diseases.Front Microbiol. 2022 Dec 14;13:1034537. doi: 10.3389/fmicb.2022.1034537. eCollection 2022. Front Microbiol. 2022. PMID: 36590426 Free PMC article. Review.
-
The Microbial Metabolite Trimethylamine N-Oxide Links Vascular Dysfunctions and the Autoimmune Disease Rheumatoid Arthritis.Nutrients. 2019 Aug 7;11(8):1821. doi: 10.3390/nu11081821. Nutrients. 2019. PMID: 31394758 Free PMC article. Review.
-
The relationship between trimethylamine-N-oxide and the risk of acute ischemic stroke: A dose‒response meta-analysis.PLoS One. 2023 Oct 26;18(10):e0293275. doi: 10.1371/journal.pone.0293275. eCollection 2023. PLoS One. 2023. PMID: 37883346 Free PMC article.

