New Perspectives on Roles of Alpha-Synuclein in Parkinson's Disease
- PMID: 30524265
- PMCID: PMC6261981
- DOI: 10.3389/fnagi.2018.00370
New Perspectives on Roles of Alpha-Synuclein in Parkinson's Disease
Abstract
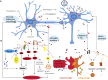
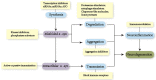
Parkinson's disease (PD) is one of the synucleinopathies spectrum of disorders typified by the presence of intraneuronal protein inclusions. It is primarily composed of misfolded and aggregated forms of alpha-synuclein (α-syn), the toxicity of which has been attributed to the transition from an α-helical conformation to a β-sheetrich structure that polymerizes to form toxic oligomers. This could spread and initiate the formation of "LB-like aggregates," by transcellular mechanisms with seeding and subsequent permissive templating. This hypothesis postulates that α-syn is a prion-like pathological agent and responsible for the progression of Parkinson's pathology. Moreover, the involvement of the inflammatory response in PD pathogenesis has been reported on the excessive microglial activation and production of pro-inflammatory cytokines. At last, we describe several treatment approaches that target the pathogenic α-syn protein, especially the oligomers, which are currently being tested in advanced animal experiments or are already in clinical trials. However, there are current challenges with therapies that target α-syn, for example, difficulties in identifying varying α-syn conformations within different individuals as well as both the cost and need of long-duration large trials.
Keywords: Parkinson’s disease; alpha-synuclein; neurodegeneration; neurotherapy; prion-like.
Figures

Similar articles
-
Parkinson's disease and alpha synuclein: is Parkinson's disease a prion-like disorder?Mov Disord. 2013 Jan;28(1):31-40. doi: 10.1002/mds.25373. Mov Disord. 2013. PMID: 23390095 Review.
-
New therapeutic approaches to target alpha-synuclein in Parkinson's disease: The role of immunotherapy.Int Rev Neurobiol. 2019;146:281-295. doi: 10.1016/bs.irn.2019.06.014. Epub 2019 Jul 18. Int Rev Neurobiol. 2019. PMID: 31349931 Review.
-
Detecting Alpha Synuclein Seeding Activity in Formaldehyde-Fixed MSA Patient Tissue by PMCA.Mol Neurobiol. 2018 Nov;55(11):8728-8737. doi: 10.1007/s12035-018-1007-y. Epub 2018 Mar 27. Mol Neurobiol. 2018. PMID: 29589283 Free PMC article.
-
Bent out of shape: α-Synuclein misfolding and the convergence of pathogenic pathways in Parkinson's disease.FEBS Lett. 2015 Dec 21;589(24 Pt A):3749-59. doi: 10.1016/j.febslet.2015.10.023. Epub 2015 Oct 23. FEBS Lett. 2015. PMID: 26505673 Free PMC article. Review.
-
FcγRIIB mediates the inhibitory effect of aggregated α-synuclein on microglial phagocytosis.Neurobiol Dis. 2015 Nov;83:90-9. doi: 10.1016/j.nbd.2015.08.025. Epub 2015 Sep 3. Neurobiol Dis. 2015. PMID: 26342897
Cited by
-
Brain-on-a-chip: an emerging platform for studying the nanotechnology-biology interface for neurodegenerative disorders.J Nanobiotechnology. 2024 Sep 18;22(1):573. doi: 10.1186/s12951-024-02720-0. J Nanobiotechnology. 2024. PMID: 39294645 Free PMC article. Review.
-
Cellular and Molecular Events Leading to Paraquat-Induced Apoptosis: Mechanistic Insights into Parkinson's Disease Pathophysiology.Mol Neurobiol. 2022 Jun;59(6):3353-3369. doi: 10.1007/s12035-022-02799-2. Epub 2022 Mar 19. Mol Neurobiol. 2022. PMID: 35306641 Free PMC article. Review.
-
Astrocytic Oxidative/Nitrosative Stress Contributes to Parkinson's Disease Pathogenesis: The Dual Role of Reactive Astrocytes.Antioxidants (Basel). 2019 Aug 1;8(8):265. doi: 10.3390/antiox8080265. Antioxidants (Basel). 2019. PMID: 31374936 Free PMC article. Review.
-
Nanoparticle-based Gene Therapy for Neurodegenerative Disorders.Mini Rev Med Chem. 2024;24(19):1723-1745. doi: 10.2174/0113895575301011240407082559. Mini Rev Med Chem. 2024. PMID: 38676491 Review.
-
Alterations in RNA editing in skeletal muscle following exercise training in individuals with Parkinson's disease.PLoS One. 2023 Dec 22;18(12):e0287078. doi: 10.1371/journal.pone.0287078. eCollection 2023. PLoS One. 2023. PMID: 38134032 Free PMC article.
References
-
- Alegre-Abarrategui J., Christian H., Lufino M. M., Mutihac R., Venda L. L., Ansorge O., et al. (2009). LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum. Mol. Genet. 18 4022–4034. 10.1093/hmg/ddp346 - DOI - PMC - PubMed
-
- Alzforum (2017). α-Synuclein Antibodies Enter Phase 2, Sans Biomarker. Available: https://www.alzforum.org/news/conference-coverage/synuclein-antibodies-e...
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

