Peroxisome biogenesis deficiency attenuates the BDNF-TrkB pathway-mediated development of the cerebellum
- PMID: 30519675
- PMCID: PMC6277683
- DOI: 10.26508/lsa.201800062
Peroxisome biogenesis deficiency attenuates the BDNF-TrkB pathway-mediated development of the cerebellum
Abstract
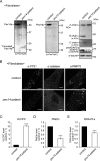
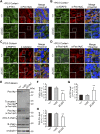
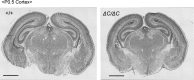
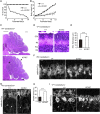
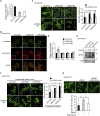
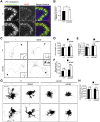
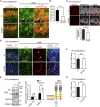
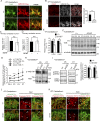
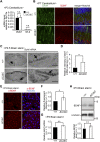
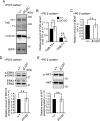
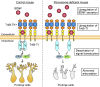
Peroxisome biogenesis disorders (PBDs) manifest as neurological deficits in the central nervous system, including neuronal migration defects and abnormal cerebellum development. However, the mechanisms underlying pathogenesis remain enigmatic. Here, to investigate how peroxisome deficiency causes neurological defects of PBDs, we established a new PBD model mouse defective in peroxisome assembly factor Pex14p, termed Pex14 ΔC/ΔC mouse. Pex14 ΔC/ΔC mouse manifests a severe symptom such as disorganization of cortical laminar structure and dies shortly after birth, although peroxisomal biogenesis and metabolism are partially defective. The Pex14 ΔC/ΔC mouse also shows malformation of the cerebellum including the impaired dendritic development of Purkinje cells. Moreover, extracellular signal-regulated kinase and AKT signaling are attenuated in this mutant mouse by an elevated level of brain-derived neurotrophic factor (BDNF) together with the enhanced expression of TrkB-T1, a dominant-negative isoform of the BDNF receptor. Our results suggest that dysregulation of the BDNF-TrkB pathway, an essential signaling for cerebellar morphogenesis, gives rise to the pathogenesis of the cerebellum in PBDs.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
A peroxisome deficiency-induced reductive cytosol state up-regulates the brain-derived neurotrophic factor pathway.J Biol Chem. 2020 Apr 17;295(16):5321-5334. doi: 10.1074/jbc.RA119.011989. Epub 2020 Mar 12. J Biol Chem. 2020. PMID: 32165495 Free PMC article.
-
Peroxisome Deficiency Impairs BDNF Signaling and Memory.Front Cell Dev Biol. 2020 Oct 14;8:567017. doi: 10.3389/fcell.2020.567017. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33163488 Free PMC article.
-
Recent insights into peroxisome biogenesis and associated diseases.J Cell Sci. 2020 May 11;133(9):jcs236943. doi: 10.1242/jcs.236943. J Cell Sci. 2020. PMID: 32393673 Review.
-
Molecular insights into peroxisome homeostasis and peroxisome biogenesis disorders.Biochim Biophys Acta Mol Cell Res. 2022 Nov;1869(11):119330. doi: 10.1016/j.bbamcr.2022.119330. Epub 2022 Jul 30. Biochim Biophys Acta Mol Cell Res. 2022. PMID: 35917894 Review.
-
BDNF signaling in the rat cerebello-vestibular pathway during vestibular compensation: BDNF signaling in vestibular compensation.FEBS J. 2015 Sep;282(18):3579-91. doi: 10.1111/febs.13360. Epub 2015 Jul 18. FEBS J. 2015. PMID: 26111610
Cited by
-
A peroxisome deficiency-induced reductive cytosol state up-regulates the brain-derived neurotrophic factor pathway.J Biol Chem. 2020 Apr 17;295(16):5321-5334. doi: 10.1074/jbc.RA119.011989. Epub 2020 Mar 12. J Biol Chem. 2020. PMID: 32165495 Free PMC article.
-
Mammalian Homologue NME3 of DYNAMO1 Regulates Peroxisome Division.Int J Mol Sci. 2020 Oct 28;21(21):8040. doi: 10.3390/ijms21218040. Int J Mol Sci. 2020. PMID: 33126676 Free PMC article.
-
Peroxisome Deficiency Impairs BDNF Signaling and Memory.Front Cell Dev Biol. 2020 Oct 14;8:567017. doi: 10.3389/fcell.2020.567017. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33163488 Free PMC article.
-
Peroxisomal homeostasis in metabolic diseases and its implication in ferroptosis.Cell Commun Signal. 2024 Oct 4;22(1):475. doi: 10.1186/s12964-024-01862-w. Cell Commun Signal. 2024. PMID: 39367496 Free PMC article. Review.
-
Mouse Models to Study Peroxisomal Functions and Disorders: Overview, Caveats, and Recommendations.Methods Mol Biol. 2023;2643:469-500. doi: 10.1007/978-1-0716-3048-8_34. Methods Mol Biol. 2023. PMID: 36952207
References
-
- Abe Y, Honsho M, Nakanishi H, Taguchi R, Fujiki Y (2014) Very-long-chain polyunsaturated fatty acids accumulate in phosphatidylcholine of fibroblasts from patients with Zellweger syndrome and acyl-CoA oxidase1 deficiency. Biochim Biophys Acta 1841: 610–619. 10.1016/j.bbalip.2014.01.001 - DOI - PubMed
-
- Baranowska-Bosiacka I, Struzynska L, Gutowska I, Machalinska A, Kolasa A, Klos P, Czapski GA, Kurzawski M, Prokopowicz A, Marchlewicz M, et al. (2013) Perinatal exposure to lead induces morphological, ultrastructural and molecular alterations in the hippocampus. Toxicology 303: 187–200. 10.1016/j.tox.2012.10.027 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
