NLRP3 lacking the leucine-rich repeat domain can be fully activated via the canonical inflammasome pathway
- PMID: 30518920
- PMCID: PMC6281599
- DOI: 10.1038/s41467-018-07573-4
NLRP3 lacking the leucine-rich repeat domain can be fully activated via the canonical inflammasome pathway
Abstract
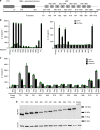
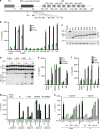
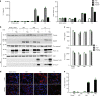
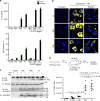
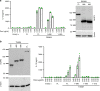
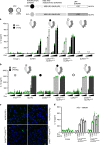
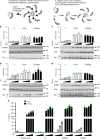
NLRP3 is a cytosolic sensor triggered by different pathogen- and self-derived signals that plays a central role in a variety of pathological conditions, including sterile inflammation. The leucine-rich repeat domain is present in several innate immune receptors, where it is frequently responsible for sensing danger signals and regulation of activation. Here we show by reconstitution of truncated and chimeric variants into Nlrp3-/- macrophages that the leucine-rich repeat domain is dispensable for activation and self-regulation of NLRP3 by several different triggers. The pyrin domain on the other hand is required to maintain NLRP3 in the inactive conformation. A fully responsive minimal NLRP3 truncation variant reconstitutes peritonitis in Nlrp3-/- mice. We demonstrate that in contrast to pathogen-activated NLRC4, the constitutively active NLRP3 molecule cannot engage wild-type NLRP3 molecules in a self-catalytic oligomerization. This lack of signal amplification is likely a protective mechanism to decrease sensitivity to endogenous triggers to impede autoinflammation.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
β-arrestin1 is critical for the full activation of NLRP3 and NLRC4 inflammasomes.J Immunol. 2015 Feb 15;194(4):1867-73. doi: 10.4049/jimmunol.1401989. Epub 2015 Jan 12. J Immunol. 2015. PMID: 25582856
-
Bacterial Outer Membrane Vesicle-Mediated Cytosolic Delivery of Flagellin Triggers Host NLRC4 Canonical Inflammasome Signaling.Front Immunol. 2020 Nov 18;11:581165. doi: 10.3389/fimmu.2020.581165. eCollection 2020. Front Immunol. 2020. PMID: 33312172 Free PMC article.
-
The lncRNA Neat1 promotes activation of inflammasomes in macrophages.Nat Commun. 2019 Apr 2;10(1):1495. doi: 10.1038/s41467-019-09482-6. Nat Commun. 2019. PMID: 30940803 Free PMC article.
-
Exogenous nanoparticles and endogenous crystalline molecules as danger signals for the NLRP3 inflammasomes.J Cell Physiol. 2019 May;234(5):5436-5450. doi: 10.1002/jcp.27475. Epub 2018 Oct 28. J Cell Physiol. 2019. PMID: 30370619 Review.
-
Mechanism and Regulation of NLRP3 Inflammasome Activation.Trends Biochem Sci. 2016 Dec;41(12):1012-1021. doi: 10.1016/j.tibs.2016.09.002. Epub 2016 Sep 23. Trends Biochem Sci. 2016. PMID: 27669650 Free PMC article. Review.
Cited by
-
Inflammasomes: Threat-Assessment Organelles of the Innate Immune System.Immunity. 2019 Oct 15;51(4):609-624. doi: 10.1016/j.immuni.2019.08.005. Epub 2019 Aug 28. Immunity. 2019. PMID: 31473100 Free PMC article. Review.
-
Alternative splicing regulates stochastic NLRP3 activity.Nat Commun. 2019 Jul 19;10(1):3238. doi: 10.1038/s41467-019-11076-1. Nat Commun. 2019. PMID: 31324763 Free PMC article.
-
FMDV Leader Protein Interacts with the NACHT and LRR Domains of NLRP3 to Promote IL-1β Production.Viruses. 2021 Dec 23;14(1):22. doi: 10.3390/v14010022. Viruses. 2021. PMID: 35062226 Free PMC article.
-
Therapeutic regulation of the NLRP3 inflammasome in chronic inflammatory diseases.Arch Pharm Res. 2021 Jan;44(1):16-35. doi: 10.1007/s12272-021-01307-9. Epub 2021 Feb 3. Arch Pharm Res. 2021. PMID: 33534121 Free PMC article. Review.
-
Epithelial Nlrp10 inflammasome mediates protection against intestinal autoinflammation.Nat Immunol. 2023 Apr;24(4):585-594. doi: 10.1038/s41590-023-01450-z. Epub 2023 Mar 20. Nat Immunol. 2023. PMID: 36941399
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

