SOX2 in cancer stemness: tumor malignancy and therapeutic potentials
- PMID: 30517668
- PMCID: PMC7109607
- DOI: 10.1093/jmcb/mjy080
SOX2 in cancer stemness: tumor malignancy and therapeutic potentials
Abstract
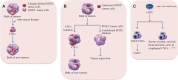
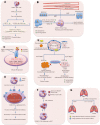
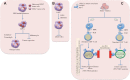
Cancer stem cells (CSCs), a minor subpopulation of tumor bulks with self-renewal and seeding capacity to generate new tumors, posit a significant challenge to develop effective and long-lasting anti-cancer therapies. The emergence of drug resistance appears upon failure of chemo-/radiation therapy to eradicate the CSCs, thereby leading to CSC-mediated clinical relapse. Accumulating evidence suggests that transcription factor SOX2, a master regulator of embryonic and induced pluripotent stem cells, drives cancer stemness, fuels tumor initiation, and contributes to tumor aggressiveness through major drug resistance mechanisms like epithelial-to-mesenchymal transition, ATP-binding cassette drug transporters, anti-apoptotic and/or pro-survival signaling, lineage plasticity, and evasion of immune surveillance. Gaining a better insight and comprehensive interrogation into the mechanistic basis of SOX2-mediated generation of CSCs and treatment failure might therefore lead to new therapeutic targets involving CSC-specific anti-cancer strategies.
Keywords: SOX2; cancer stem cells (CSCs); drug resistance; therapeutic potentials.
© The Author(s) (2019). Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, IBCB, SIBS, CAS.
Figures
Similar articles
-
Stem cell programs in cancer initiation, progression, and therapy resistance.Theranostics. 2020 Jul 9;10(19):8721-8743. doi: 10.7150/thno.41648. eCollection 2020. Theranostics. 2020. PMID: 32754274 Free PMC article. Review.
-
XIAP Limits Autophagic Degradation of Sox2 and Is A Therapeutic Target in Nasopharyngeal Carcinoma Stem Cells.Theranostics. 2018 Feb 5;8(6):1494-1510. doi: 10.7150/thno.21717. eCollection 2018. Theranostics. 2018. PMID: 29556337 Free PMC article.
-
Advances in Therapeutic Targeting of Cancer Stem Cells within the Tumor Microenvironment: An Updated Review.Cells. 2020 Aug 13;9(8):1896. doi: 10.3390/cells9081896. Cells. 2020. PMID: 32823711 Free PMC article. Review.
-
Regulation of Head and Neck Squamous Cancer Stem Cells by PI3K and SOX2.J Natl Cancer Inst. 2016 Sep 15;109(1):djw189. doi: 10.1093/jnci/djw189. Print 2017 Jan. J Natl Cancer Inst. 2016. PMID: 27634934 Free PMC article.
-
Cancer stem cell (CSC) resistance drivers.Life Sci. 2019 Oct 1;234:116781. doi: 10.1016/j.lfs.2019.116781. Epub 2019 Aug 17. Life Sci. 2019. PMID: 31430455 Review.
Cited by
-
6-Methoxymellein Isolated from Carrot (Daucus carota L.) Targets Breast Cancer Stem Cells by Regulating NF-κB Signaling.Molecules. 2020 Sep 23;25(19):4374. doi: 10.3390/molecules25194374. Molecules. 2020. PMID: 32977636 Free PMC article.
-
SOX2 Expression Does Not Guarantee Cancer Stem Cell-like Characteristics in Lung Adenocarcinoma.Cells. 2024 Jan 24;13(3):216. doi: 10.3390/cells13030216. Cells. 2024. PMID: 38334608 Free PMC article.
-
Pleiotropic effects of DCLK1 in cancer and cancer stem cells.Front Mol Biosci. 2022 Sep 26;9:965730. doi: 10.3389/fmolb.2022.965730. eCollection 2022. Front Mol Biosci. 2022. PMID: 36250024 Free PMC article. Review.
-
Self-Renewal and Pluripotency in Osteosarcoma Stem Cells' Chemoresistance: Notch, Hedgehog, and Wnt/β-Catenin Interplay with Embryonic Markers.Int J Mol Sci. 2023 May 7;24(9):8401. doi: 10.3390/ijms24098401. Int J Mol Sci. 2023. PMID: 37176108 Free PMC article. Review.
-
CD24+CD44+CD54+EpCAM+ gastric cancer stem cells predict tumor progression and metastasis: clinical and experimental evidence.Stem Cell Res Ther. 2023 Feb 3;14(1):16. doi: 10.1186/s13287-023-03241-7. Stem Cell Res Ther. 2023. PMID: 36737794 Free PMC article.
References
-
- Annovazzi L., Mellai M., Caldera V., et al. . (2011). SOX2 expression and amplification in gliomas and glioma cell lines. Cancer Genomics Proteomics 8, 139–147. - PubMed
-
- Balsara B.R., Sonoda G., du Manoir S., et al. . (1997). Comparative genomic hybridization analysis detects frequent, often high-level, overrepresentation of DNA sequences at 3q, 5p, 7p, and 8q in human non-small cell lung carcinomas. Cancer Res. 57, 2116–2120. - PubMed

