Phenotypic and Functional Profiles of Antigen-Specific CD4+ and CD8+ T Cells Associated With Infection Control in Patients With Cutaneous Leishmaniasis
- PMID: 30510917
- PMCID: PMC6252334
- DOI: 10.3389/fcimb.2018.00393
Phenotypic and Functional Profiles of Antigen-Specific CD4+ and CD8+ T Cells Associated With Infection Control in Patients With Cutaneous Leishmaniasis
Abstract
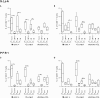
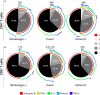
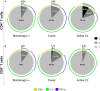
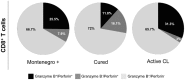
The host immunological response is a key factor determining the pathogenesis of cutaneous leishmaniasis. It is known that a Th1 cellular response is associated with infection control and that antigen-specific memory T cells are necessary for the development of a rapid and strong protective cellular response. The present manuscript reports the analysis of the functional and phenotypic profiles of antigen-specific CD4+ and CD8+ T cells from patients cured of cutaneous leishmaniasis (CL), patients with an active process of cutaneous leishmaniasis, asymptomatic individuals with a positive Montenegro test and healthy donors (HD). Peripheral blood mononuclear cells (PBMCs) from the patients exhibited a lymphoproliferative capacity after stimulation with total soluble protein from either Leishmania panamensis (SLpA) or Leishmania infantum (SLiA) or with a recombinant paraflagellar rod protein-1 (rPFR1). Higher frequencies of antigen-specific TNAIVE cells, mainly following stimulation with rPFR1, were observed in asymptomatic and cured patients than in patients with active cutaneous leishmaniasis, while T cells from patients with active cutaneous leishmaniasis showed a higher percentage of effector memory T cells (TEM for CD4+ T cells and TEMRA for CD8+ T cells). The amount of antigen-specific CD57+/CD8+ TEMRA cells in patients with active cutaneous leishmaniasis was higher than that in cured patients and asymptomatic subjects. Regarding functionality, a more robust multifunctional CD8+ T cell response was detected in cured patients than in those with active cutaneous leishmaniasis. Moreover, cured patients showed a significant increase in the frequency of cells expressing a Th1-type cytotoxic production profile (IFN-γ+/granzyme-B/+perforin+). Patients with an active leishmaniosis process had a significantly higher frequency of CD8+ T cells expressing the inhibitory CD160 and 2B4 receptors than did cured patients. The expression profile observed in cured patients could be indicative of an imbalance toward a CD8+ Th1 response, which could be associated with infection control; consequently, the determination of this profile could be a useful tool for facilitating the clinical follow-up of patients with cutaneous leishmaniasis. The results also suggest a possible exhaustion process of CD8+ T cells associated with the evolution of Leishmania infection.
Keywords: CD8+ and CD4+ T-cells; Leishmania; Th1-cytokines; biomarkers; inhibitory receptors; leishmaniasis; paraflagellar rod protein-1; phenotype.
Figures

Similar articles
-
Design of multi-epitope peptides containing HLA class-I and class-II-restricted epitopes derived from immunogenic Leishmania proteins, and evaluation of CD4+ and CD8+ T cell responses induced in cured cutaneous leishmaniasis subjects.PLoS Negl Trop Dis. 2020 Mar 16;14(3):e0008093. doi: 10.1371/journal.pntd.0008093. eCollection 2020 Mar. PLoS Negl Trop Dis. 2020. PMID: 32176691 Free PMC article.
-
Flow cytometric determination of cellular sources and frequencies of key cytokine-producing lymphocytes directed against recombinant LACK and soluble Leishmania antigen in human cutaneous leishmaniasis.Infect Immun. 2001 May;69(5):3232-9. doi: 10.1128/IAI.69.5.3232-3239.2001. Infect Immun. 2001. PMID: 11292745 Free PMC article.
-
Antigenicity of Leishmania-Activated C-Kinase Antigen (LACK) in Human Peripheral Blood Mononuclear Cells, and Protective Effect of Prime-Boost Vaccination With pCI-neo-LACK Plus Attenuated LACK-Expressing Vaccinia Viruses in Hamsters.Front Immunol. 2018 Apr 23;9:843. doi: 10.3389/fimmu.2018.00843. eCollection 2018. Front Immunol. 2018. PMID: 29740446 Free PMC article.
-
[Immunopathology of American tegumentary leishmaniasis].Acta Cient Venez. 1998;49(1):42-56. Acta Cient Venez. 1998. PMID: 10205916 Review. Spanish.
-
Review on the Role of Host Immune Response in Protection and Immunopathogenesis during Cutaneous Leishmaniasis Infection.J Immunol Res. 2020 Jun 18;2020:2496713. doi: 10.1155/2020/2496713. eCollection 2020. J Immunol Res. 2020. PMID: 32656269 Free PMC article. Review.
Cited by
-
PD-1 Blockade Modulates Functional Activities of Exhausted-Like T Cell in Patients With Cutaneous Leishmaniasis.Front Immunol. 2021 Mar 9;12:632667. doi: 10.3389/fimmu.2021.632667. eCollection 2021. Front Immunol. 2021. PMID: 33767700 Free PMC article.
-
Leishmania donovani Impedes Antileishmanial Immunity by Suppressing Dendritic Cells via the TIM-3 Receptor.mBio. 2022 Aug 30;13(4):e0330921. doi: 10.1128/mbio.03309-21. Epub 2022 Aug 4. mBio. 2022. PMID: 35924848 Free PMC article.
-
Memory T cells: promising biomarkers for evaluating protection and vaccine efficacy against leishmaniasis.Front Immunol. 2024 Feb 26;15:1304696. doi: 10.3389/fimmu.2024.1304696. eCollection 2024. Front Immunol. 2024. PMID: 38469319 Free PMC article. Review.
-
The Role of Senescent CD8+T Cells in the Pathogenesis of Disseminated Leishmaniasis.Pathogens. 2024 May 29;13(6):460. doi: 10.3390/pathogens13060460. Pathogens. 2024. PMID: 38921758 Free PMC article.
-
Revival of Leishmanization and Leishmanin.Front Cell Infect Microbiol. 2021 Mar 17;11:639801. doi: 10.3389/fcimb.2021.639801. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33816344 Free PMC article. Review.
References
-
- Barroso D. H., Falcão S. A. C., da Motta J. O. C., Sevilha Dos Santos L., Takano G. H. S., Gomes C. M., et al. . (2018). PD-L1 may mediate T-cell exhaustion in a case of early diffuse leishmaniasis caused by Leishmania (L.) amazonensis. Front. Immunol. 9:1021. 10.3389/fimmu.2018.01021 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

