Alpha-tubulin acetyltransferase/MEC-17 regulates cancer cell migration and invasion through epithelial-mesenchymal transition suppression and cell polarity disruption
- PMID: 30504808
- PMCID: PMC6269487
- DOI: 10.1038/s41598-018-35392-6
Alpha-tubulin acetyltransferase/MEC-17 regulates cancer cell migration and invasion through epithelial-mesenchymal transition suppression and cell polarity disruption
Abstract
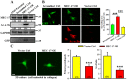
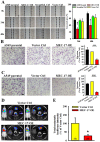
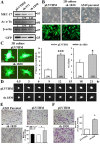
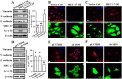
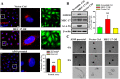
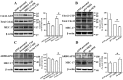
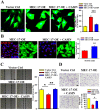
MEC-17, a newly identified alpha-tubulin-N-acetyltransferase 1, serves as the major α-tubulin acetyltransferase to promote α-tubulin acetylation in vitro and in vivo. Alteration of α-tubulin acetylation may be involved in morphology regulation, cell migration, and tumour metastasis. However, MEC-17's role in cell physiology and its effect on epithelial-mesenchymal transition (EMT) and cell polarity remain elusive. In the present study, we characterized the overexpressed or downregulated cell models through gene targeting as MEC-17 gain- or loss-of-function. Overexpression of MEC-17 enhanced the cell spreading area, suppressed pseudopods formation in a three-dimensional (3D) culture system, and inhibited cancer cell migratory and invasive ability and tumour metastasis by orthotopic lung cancer animal model. Furthermore, morphological change and migration inhibition of cancer cells were accompanied by EMT repression, Golgi reorientation, and polarity disruption caused by alteration of cdc42 activity via a decrease in Rho-GAP, ARHGAP21. By contrast, a reduction in endogenous MEC-17 accelerated the pseudopods formation and EMT, and facilitated cell migration and invasion. These results demonstrated the crucial role of MEC-17 in the modulation of intrinsic cell morphogenesis, migration, and invasive function through regulation of EMT and cell polarity.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
MEC-17 deficiency leads to reduced α-tubulin acetylation and impaired migration of cortical neurons.J Neurosci. 2012 Sep 12;32(37):12673-83. doi: 10.1523/JNEUROSCI.0016-12.2012. J Neurosci. 2012. PMID: 22972992 Free PMC article.
-
ATAT1/MEC-17 acetyltransferase and HDAC6 deacetylase control a balance of acetylation of alpha-tubulin and cortactin and regulate MT1-MMP trafficking and breast tumor cell invasion.Eur J Cell Biol. 2012 Nov-Dec;91(11-12):950-60. doi: 10.1016/j.ejcb.2012.07.001. Epub 2012 Aug 16. Eur J Cell Biol. 2012. PMID: 22902175
-
ARHGAP21 protein, a new partner of α-tubulin involved in cell-cell adhesion formation and essential for epithelial-mesenchymal transition.J Biol Chem. 2013 Jan 25;288(4):2179-89. doi: 10.1074/jbc.M112.432716. Epub 2012 Dec 12. J Biol Chem. 2013. PMID: 23235160 Free PMC article.
-
Cell polarity signaling in the plasticity of cancer cell invasiveness.Oncotarget. 2016 May 3;7(18):25022-49. doi: 10.18632/oncotarget.7214. Oncotarget. 2016. PMID: 26872368 Free PMC article. Review.
-
Cell polarity in morphogenesis and metastasis.Philos Trans R Soc Lond B Biol Sci. 2013 Sep 23;368(1629):20130012. doi: 10.1098/rstb.2013.0012. Print 2013. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 24062582 Free PMC article. Review.
Cited by
-
LEDGF/p75 Is Required for an Efficient DNA Damage Response.Int J Mol Sci. 2021 May 30;22(11):5866. doi: 10.3390/ijms22115866. Int J Mol Sci. 2021. PMID: 34070855 Free PMC article.
-
Regulatory roles of NAT10 in airway epithelial cell function and metabolism in pathological conditions.Cell Biol Toxicol. 2023 Aug;39(4):1237-1256. doi: 10.1007/s10565-022-09743-z. Epub 2022 Jul 25. Cell Biol Toxicol. 2023. PMID: 35877022
-
Intrinsic and Extrinsic Factors Affecting Microtubule Dynamics in Normal and Cancer Cells.Molecules. 2020 Aug 14;25(16):3705. doi: 10.3390/molecules25163705. Molecules. 2020. PMID: 32823874 Free PMC article. Review.
-
Perphenazine and prochlorperazine decrease glioblastoma U-87 MG cell migration and invasion: Analysis of the ABCB1 and ABCG2 transporters, E-cadherin, α-tubulin and integrins (α3, α5, and β1) levels.Oncol Lett. 2022 Jun;23(6):182. doi: 10.3892/ol.2022.13302. Epub 2022 Apr 15. Oncol Lett. 2022. PMID: 35527777 Free PMC article.
-
Cytoskeleton Dynamics in Peripheral T Cell Lymphomas: An Intricate Network Sustaining Lymphomagenesis.Front Oncol. 2021 Apr 13;11:643620. doi: 10.3389/fonc.2021.643620. eCollection 2021. Front Oncol. 2021. PMID: 33928032 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- MOST 103-2320-B-006-036-MY3/Ministry of Science and Technology, Taiwan (Ministry of Science and Technology of Taiwan)/International
- MOST 103-2325-B-400-012/Ministry of Science and Technology, Taiwan (Ministry of Science and Technology of Taiwan)/International
- MOST 104-2325-B-400 -002/Ministry of Science and Technology, Taiwan (Ministry of Science and Technology of Taiwan)/International
- MOST 105-2325-B-400-001/Ministry of Science and Technology, Taiwan (Ministry of Science and Technology of Taiwan)/International
- MOST 106-2314-B-006-076-MY3/Ministry of Science and Technology, Taiwan (Ministry of Science and Technology of Taiwan)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

