The function of miR-143, miR-145 and the MiR-143 host gene in cardiovascular development and disease
- PMID: 30502421
- PMCID: PMC6395947
- DOI: 10.1016/j.vph.2018.11.006
The function of miR-143, miR-145 and the MiR-143 host gene in cardiovascular development and disease
Abstract
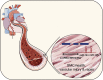
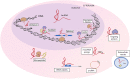
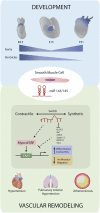
Noncoding RNAs (long noncoding RNAs and small RNAs) are emerging as critical modulators of phenotypic changes associated with physiological and pathological contexts in a variety of cardiovascular diseases (CVDs). Although it has been well established that hereditable genetic alterations and exposure to risk factors are crucial in the development of CVDs, other critical regulators of cell function impact on disease processes. Here we discuss noncoding RNAs have only recently been identified as key players involved in the progression of disease. In particular, we discuss micro RNA (miR)-143/145 since they represent one of the most characterised microRNA clusters regulating smooth muscle cell (SMC) differentiation and phenotypic switch in response to vascular injury and remodelling. MiR143HG is a well conserved long noncoding RNA (lncRNA), which is the host gene for miR-143/145 and recently implicated in cardiac specification during heart development. Although the lncRNA-miRNA interactions have not been completely characterised, their crosstalk is now beginning to emerge and likely requires further research focus. In this review we give an overview of the biology of the genomic axis that is miR-143/145 and MiR143HG, focusing on their important functional role(s) in the cardiovascular system.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Long noncoding RNAs and microRNAs in cardiovascular pathophysiology.Circ Res. 2015 Feb 13;116(4):751-62. doi: 10.1161/CIRCRESAHA.116.303549. Circ Res. 2015. PMID: 25677521 Review.
-
Super-enhancer lncs to cardiovascular development and disease.Biochim Biophys Acta. 2016 Jul;1863(7 Pt B):1953-60. doi: 10.1016/j.bbamcr.2015.11.026. Epub 2015 Nov 24. Biochim Biophys Acta. 2016. PMID: 26620798 Review.
-
The novel regulatory role of lncRNA-miRNA-mRNA axis in cardiovascular diseases.J Cell Mol Med. 2018 Dec;22(12):5768-5775. doi: 10.1111/jcmm.13866. Epub 2018 Sep 6. J Cell Mol Med. 2018. PMID: 30188595 Free PMC article. Review.
-
Long noncoding RNAs in cardiovascular diseases.Circ Res. 2015 Feb 13;116(4):737-50. doi: 10.1161/CIRCRESAHA.116.302521. Circ Res. 2015. PMID: 25677520 Review.
-
Long Noncoding Competing Endogenous RNA Networks in Age-Associated Cardiovascular Diseases.Int J Mol Sci. 2019 Jun 24;20(12):3079. doi: 10.3390/ijms20123079. Int J Mol Sci. 2019. PMID: 31238513 Free PMC article. Review.
Cited by
-
Protective Roles and Therapeutic Effects of Gallic Acid in the Treatment of Cardiovascular Diseases: Current Trends and Future Directions.Curr Med Chem. 2024;31(24):3733-3751. doi: 10.2174/0109298673259299230921150030. Curr Med Chem. 2024. PMID: 37815180 Review.
-
Genetic Variants Identified by Whole Exome Sequencing in a Large Italian Family with High Plasma Levels of Factor VIII and Von Willebrand Factor.Int J Mol Sci. 2023 Sep 15;24(18):14167. doi: 10.3390/ijms241814167. Int J Mol Sci. 2023. PMID: 37762470 Free PMC article.
-
N6-methyladenosine-driven miR-143/145-KLF4 circuit orchestrates the phenotypic switch of pulmonary artery smooth muscle cells.Cell Mol Life Sci. 2024 Jun 12;81(1):256. doi: 10.1007/s00018-024-05304-1. Cell Mol Life Sci. 2024. PMID: 38866991 Free PMC article.
-
Dysregulation of tissue and serum microRNAs in organ transplant recipients with cutaneous squamous cell carcinomas.Health Sci Rep. 2020 Nov 19;3(4):e205. doi: 10.1002/hsr2.205. eCollection 2020 Dec. Health Sci Rep. 2020. PMID: 33251338 Free PMC article.
-
Identification of Long Noncoding RNAs Involved in Differentiation and Survival of Vascular Smooth Muscle Cells.Mol Ther Nucleic Acids. 2020 Aug 29;22:209-221. doi: 10.1016/j.omtn.2020.08.032. eCollection 2020 Dec 4. Mol Ther Nucleic Acids. 2020. PMID: 33230428 Free PMC article.
References
-
- Collins F.S., Lander E.S., Rogers J., Waterson R.H. Finishing the euchromatic sequence of the human genome. Nature. 2004;431:931–945. - PubMed
-
- Consortium T. Fantom. The transcriptional landscape of the mammalian genome. Science. 2006;309:1559–1563. - PubMed
-
- Kim V.N., Han J., Siomi M.C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 2009;10:126–139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

