Exploring reporting quality of systematic reviews and Meta-analyses on nursing interventions in patients with Alzheimer's disease before and after PRISMA introduction
- PMID: 30497417
- PMCID: PMC6267794
- DOI: 10.1186/s12874-018-0622-7
Exploring reporting quality of systematic reviews and Meta-analyses on nursing interventions in patients with Alzheimer's disease before and after PRISMA introduction
Abstract
Background: Systematic reviews (SRs) and meta-analyses (MAs) are distillation of current best available evidence, but are potentially prone to bias. The bias of SRs and MAs comes from sampling bias, selection bias and within study bias. So, their reporting quality is especially important as it may directly influence their utility for clinicians, nurses, patients and policy makers. The SRs and MAs on nursing interventions in patients with Alzheimer's disease (AD) have been increasingly published over the past decade, but the reporting quality of article has not been evaluated after the introduction of Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) Statement.
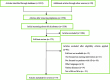
Methods: According to the inclusion and exclusion criteria, we searched the databases including PubMed, EMBASE and The Cochrane Library from inception through October 16th 2018. Two reviewers independently selected articles and extracted data. The PRISMA checklist was adopted to evaluate reporting quality. Comparisons were made between studies published before (2001-2009) and after (2011-2018) its introduction.
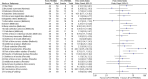
Results: A total of 77 eligible articles, 18 (23.4%) were published before the PRISMA Statement and 59 (76.6%) were published afterwards. There was higher score after publication of the PRISMA Statement than before (20.83 ± 3.78 vs 17.11 ± 4.56, P < 0.05). There was an improvement in the following items after the PRISMA statement was released (P < 0.05): title (item 1, 50.0% vs 74.6%, OR = 3.10, 95CI%: 1.00-9.61), search (item8, 27.8% vs 57.6%,OR = 3.25, 95CI%: 1.14-9.28), study selection (item 9, 44.4% vs 81.4%,OR = 6.28, 95CI%: 1.93-20.37), Data collection process (item 10, 50.0% vs 76.3%,OR = 3.45, 95CI%:1.10-10.84), risk of bias in individual studies (item 12, 50.0% vs 83.1%, OR = 5.78, 95CI%:1.71-19.52), risk of bias across studies (item15, 5.6% vs 28.8%,OR = 3.60, 95CI%:1.04-12.43), study characteristics (item 18, 77.8% vs 98.3%, OR = 28.13, 95CI%:3.35-236.19), risk of bias with studies (item 19, 50.0% vs 83.1%, OR = 5.78, 95CI%:1.71-19.52), results in individual studies (item 20, 72.2% vs 94.9%, OR = 11.09, 95CI%:1.99-61.82), conclusions (item 26, 77.8% vs 98.3%, OR = 28.13, 95CI%:3.35-236.19). After controlling for the confounding factors, there were higher PRISMA score for systematic reviews including meta-analyses, protocol or registration, can't answer of RCT, journal source of SCI (Science Citation Index), manuscript length > 13 page and funding support.
Conclusion: Since the publication of the PRISMA Statement, there has been an improvement in the quality of reporting of SRs and MAs on nursing interventions in patients with AD. More endorsement by journals of the report guideline for SRs/MAs may improve articles reporting quality, and the dissemination of reliable evidence to nurses. We recommend authors, readers, reviewers, and editors to become more acquainted with and to more strictly adhere to the PRISMA checklist.
Keywords: Alzheimer’s disease; Nursing interventions; Reporting quality; Systematic review.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable. This study does not involve human participants, human dataor human tissue.
Consent for publication
Not applicable. This study does not involve individual data.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Reporting and Methodological Quality of Systematic Reviews and Meta-Analyses of Nursing Interventions in Patients With Alzheimer's Disease: General Implications of the Findings.J Nurs Scholarsh. 2019 May;51(3):308-316. doi: 10.1111/jnu.12462. Epub 2019 Feb 25. J Nurs Scholarsh. 2019. PMID: 30806019
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
The effects of the PRISMA statement to improve the conduct and reporting of systematic reviews and meta-analyses of nursing interventions for patients with heart failure.Int J Nurs Pract. 2019 Jun;25(3):e12729. doi: 10.1111/ijn.12729. Epub 2019 Feb 20. Int J Nurs Pract. 2019. PMID: 30790391
-
Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals.Cochrane Database Syst Rev. 2012 Nov 14;11(11):MR000030. doi: 10.1002/14651858.MR000030.pub2. Cochrane Database Syst Rev. 2012. PMID: 23152285 Free PMC article. Review.
-
Reporting Quality of Systematic Reviews and Meta-Analyses of Otorhinolaryngologic Articles Based on the PRISMA Statement.PLoS One. 2015 Aug 28;10(8):e0136540. doi: 10.1371/journal.pone.0136540. eCollection 2015. PLoS One. 2015. PMID: 26317406 Free PMC article. Review.
Cited by
-
The Impact of Exercise Prescription Variables on Intervention Outcomes in Musculoskeletal Pain: An Umbrella Review of Systematic Reviews.Sports Med. 2024 Mar;54(3):711-725. doi: 10.1007/s40279-023-01966-2. Epub 2023 Dec 14. Sports Med. 2024. PMID: 38093145 Free PMC article.
-
Use of AMSTAR-2 in the methodological assessment of systematic reviews: protocol for a methodological study.Ann Transl Med. 2020 May;8(10):652. doi: 10.21037/atm-20-392a. Ann Transl Med. 2020. PMID: 32566589 Free PMC article.
-
Predictors of Dietitian Referrals in Hospitals.Nutrients. 2020 Sep 18;12(9):2863. doi: 10.3390/nu12092863. Nutrients. 2020. PMID: 32962105 Free PMC article.
-
How to make a systematic review live up to its name: perspectives from journal editors.Ann Transl Med. 2023 Jun 30;11(9):325. doi: 10.21037/atm-22-6305. Epub 2023 May 10. Ann Transl Med. 2023. PMID: 37404995 Free PMC article. No abstract available.
-
The reporting and methodological quality of systematic reviews and meta-analyses of nursing interventions for chronic obstructive pulmonary disease - A systematic review.Nurs Open. 2021 May;8(3):1489-1500. doi: 10.1002/nop2.767. Epub 2021 Jan 19. Nurs Open. 2021. PMID: 33465288 Free PMC article.
References
-
- Moher D, et al. Improving the quality of reports of Meta-analyses of randomised controlled trials: the QUOROM statement. Onkologie. 1999;23(6):597–602. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous