OK-432 Administration Inhibits Murine Allergic Rhinitis at the Induction Phase, through the Macrophage Activation with TLR2 Signaling Pathway
- PMID: 30486312
- PMCID: PMC6313634
- DOI: 10.3390/medsci6040107
OK-432 Administration Inhibits Murine Allergic Rhinitis at the Induction Phase, through the Macrophage Activation with TLR2 Signaling Pathway
Abstract
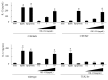
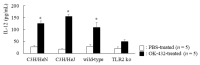
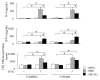
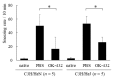
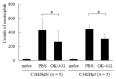
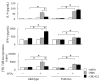
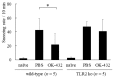
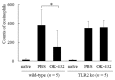
OK-432, a preparation of a low-virulence strain (Su) of Streptococcus pyogenes (Group A) killed by a penicillin and lyophilized, is a stiff inducer of Th1 cytokines, and exerts anti-cancer effects in tumor-bearing mice. OK-432 has been reported to consist of many bacterial components, such as peptidoglycan, M-protein, etc. However, it is yet to be ascertained which bacterial component induces T helper 1 (Th1) responses. For the last decade, Toll-like receptor (TLR) family proteins are well elucidated to play a role in recognizing bacterial components and inducing interleukin (IL)-12 from macrophages. Above all, peptidoglycan seems to be the agonist of TLR2 rather than the obverse. In our present study, the role of TLR2 for the recognition of OK-432 by macrophages and the effects of OK-432 are examined on murine allergic rhinitis model. Interestingly, results show IL-12 production by macrophages derived from TLR2 knock-out (ko) mice was significantly decreased, in comparison with that of macrophages derived from wild-type mice. Moreover, in TLR2 ko mice, no regulatory effect of OK-432 was observed on an allergic rhinitis model. These data indicate that TLR2 signaling is involved in regulating OK-432-induced anti-T helper 2 (Th2) immunity, and may offer a new prophylactic and therapeutic approach using OK-432 to downregulate allergic disorders, such as allergic rhinitis.
Keywords: OK-432; TLR2; allergic rhinitis; macrophage.
Conflict of interest statement
We have no conflict of interest to submit this manuscript.
Figures
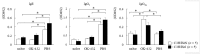
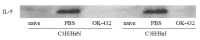
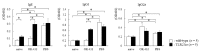

Similar articles
-
Severe impairment of anti-cancer effect of lipoteichoic acid-related molecule isolated from a penicillin-killed Streptococcus pyogenes in toll-like receptor 4-deficient mice.Int Immunopharmacol. 2001 Sep;1(9-10):1789-95. doi: 10.1016/s1567-5769(01)00103-5. Int Immunopharmacol. 2001. PMID: 11562070
-
Anti-tumor immune response induced by the fractions derived from OK-432, a streptococcal preparation, by using a monoclonal antibody TS-2 that neutralizes the interferon-gamma-inducing activity of OK-432: comparison between the TS-2-binding and TS-2-unbinding fraction.Int Immunopharmacol. 2003 May;3(5):643-55. doi: 10.1016/S1567-5769(02)00274-6. Int Immunopharmacol. 2003. PMID: 12757734
-
Antitumor effect of OK-432-derived DNA: one of the active constituents of OK-432, a streptococcal immunotherapeutic agent.J Immunother. 2006 Mar-Apr;29(2):143-50. doi: 10.1097/01.cji.0000189028.18288.6f. J Immunother. 2006. PMID: 16531815
-
Streptococcal preparation OK-432 is a potent inducer of IL-12 and a T helper cell 1 dominant state.J Immunol. 1997 Jun 15;158(12):5619-26. J Immunol. 1997. PMID: 9190909
-
Induction of Th1-type cytokines by lipoteichoic acid-related preparation isolated from OK-432, a penicillin-killed streptococcal agent.Immunopharmacology. 2000 Sep;49(3):363-76. doi: 10.1016/s0162-3109(00)00252-6. Immunopharmacology. 2000. PMID: 10996034
Cited by
-
Toll-Like Receptor Agonists as Adjuvants for Allergen Immunotherapy.Front Immunol. 2020 Nov 12;11:599083. doi: 10.3389/fimmu.2020.599083. eCollection 2020. Front Immunol. 2020. PMID: 33281825 Free PMC article. Review.
-
Total RNA and genomic DNA of Lactobacillus gasseri OLL2809 induce interleukin-12 production in the mouse macrophage cell line J774.1 via toll-like receptors 7 and 9.BMC Microbiol. 2020 Jul 20;20(1):217. doi: 10.1186/s12866-020-01900-w. BMC Microbiol. 2020. PMID: 32689952 Free PMC article.
-
The Assessment of TLR1 Gene Polymorphism Association with the Risk of Allergic Rhinitis in the Chinese Han Population from Northern China.J Asthma Allergy. 2023 Sep 18;16:979-986. doi: 10.2147/JAA.S421939. eCollection 2023. J Asthma Allergy. 2023. PMID: 37745900 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

