Immunity Against Leishmania major Infection: Parasite-Specific Granzyme B Induction as a Correlate of Protection
- PMID: 30483482
- PMCID: PMC6243638
- DOI: 10.3389/fcimb.2018.00397
Immunity Against Leishmania major Infection: Parasite-Specific Granzyme B Induction as a Correlate of Protection
Abstract
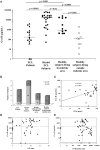
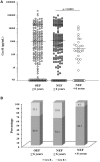
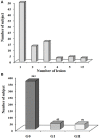
Zoonotic cutaneous leishmaniasis (ZCL) caused by Leishmania (L.) major infection is characterized by different clinical presentations which depend in part on the host factors. In attempt to investigate the impact of the host's immune response in the outcome of the disease, we conducted a prospective study of 453 individuals living in endemic foci of L. major transmission in Central Tunisia. Several factors were assessed at the baseline including (i) the presence of typical scars of ZCL, (ii) in vivo hypersensitivity reaction to leishmanin, and (iii) the in vitro release of granzyme B (Grz B) by peripheral blood mononuclear cells (PBMC) in response to stimulation with live L. major promastigotes. After one season of parasite's transmission, repeated clinical examinations allowed us to diagnose the new emerging ZCL cases. Heterogeneity was observed in terms of number of lesions developed by each individual as well as their size and spontaneous outcome, which led us to establish the parameter "severity of the disease." The efficacy of the presence of typical ZCL scar, the leishmanin skin test (LST) positive reactivity and the high levels of Grz B (≥2 ng/ml), in the protection against the development of ZCL were 29, 15, and 22%, respectively. However, these factors were more efficient against development of intermediate or severe forms of ZCL. Levels of Grz B >2 ng/ml showed the best efficacy of protection (equals to 72.8%) against development of these forms of ZCL. The association of such parameter with the positivity of the LST exhibited a better efficacy (equals to 83.6%). In conclusion, our results support the involvement of Leishmania-specific cytotoxic cellular immune response in host protection against Leishmania-infection. This factor could be of great interest in monitoring the success of vaccination against human leishmaniasis.
Keywords: Leishmania major; correlate of protection; granzyme B; re-infection; specific-cytotoxicity.
Figures


Similar articles
-
Analysis of granzyme B activity as a surrogate marker of Leishmania-specific cell-mediated cytotoxicity in zoonotic cutaneous leishmaniasis.J Infect Dis. 2004 Apr 1;189(7):1265-73. doi: 10.1086/382031. Epub 2004 Mar 12. J Infect Dis. 2004. PMID: 15031796
-
The predictive validity of naturally acquired delayed-type hypersensitivity to leishmanin in resistance to Leishmania major-associated cutaneous leishmaniasis.J Infect Dis. 2005 Dec 1;192(11):1981-7. doi: 10.1086/498042. Epub 2005 Oct 21. J Infect Dis. 2005. PMID: 16267771
-
Activated cytotoxic T cells within zoonotic cutaneous leishmaniasis lesions.Immun Inflamm Dis. 2019 Sep;7(3):95-104. doi: 10.1002/iid3.240. Epub 2019 Apr 17. Immun Inflamm Dis. 2019. PMID: 30997749 Free PMC article.
-
A review of the leishmanin skin test: A neglected test for a neglected disease.PLoS Negl Trop Dis. 2021 Jul 22;15(7):e0009531. doi: 10.1371/journal.pntd.0009531. eCollection 2021 Jul. PLoS Negl Trop Dis. 2021. PMID: 34292942 Free PMC article. Review.
-
Revival of Leishmanization and Leishmanin.Front Cell Infect Microbiol. 2021 Mar 17;11:639801. doi: 10.3389/fcimb.2021.639801. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33816344 Free PMC article. Review.
Cited by
-
Clinical and immunological spectra of human cutaneous leishmaniasis in North Africa and French Guiana.Front Immunol. 2023 Jul 27;14:1134020. doi: 10.3389/fimmu.2023.1134020. eCollection 2023. Front Immunol. 2023. PMID: 37575260 Free PMC article. Review.
-
Mastomys natalensis Has a Cellular Immune Response Profile Distinct from Laboratory Mice.Viruses. 2021 Apr 22;13(5):729. doi: 10.3390/v13050729. Viruses. 2021. PMID: 33922222 Free PMC article.
-
A Prospective cohort study of zoonotic cutaneous leishmaniasis in tunisia: Clinical and Immunological features and immune correlates of protection.PLoS Negl Trop Dis. 2023 Dec 8;17(12):e0011784. doi: 10.1371/journal.pntd.0011784. eCollection 2023 Dec. PLoS Negl Trop Dis. 2023. PMID: 38064516 Free PMC article.
-
Leishmania Major Centrin Gene-Deleted Parasites Generate Skin Resident Memory T-Cell Immune Response Analogous to Leishmanization.Front Immunol. 2022 Mar 28;13:864031. doi: 10.3389/fimmu.2022.864031. eCollection 2022. Front Immunol. 2022. PMID: 35419001 Free PMC article.
-
Unraveling the Role of Immune Checkpoints in Leishmaniasis.Front Immunol. 2021 Mar 11;12:620144. doi: 10.3389/fimmu.2021.620144. eCollection 2021. Front Immunol. 2021. PMID: 33776999 Free PMC article. Review.
References
-
- Alimohammadian M. H., Khamesipour A., Darabi H., Firooz A., Malekzadeh S., Bahonar A., et al. (2002). The role of BCG in human immune responses induced by multiple injections of autoclaved Leishmania major as a candidate vaccine against leishmaniasis. Vaccine 2, 174–180. 10.1016/S0264-410X(02)00458-9 - DOI - PubMed
-
- Ben Ismail R., Ben Rachid M. (1989). Epidemiology of leishmaniasis in Tunisia, in Tropical Communicable Diseases, ed AUPELF-UREE (Paris: John Libbey Eurotext; ), 73–80.
-
- Ben Salah A., Louzir H., Chlif S., Mokni M., Zaâtour A., Raouène M., et al. . (2005). The predictive validity of naturally acquired delayed-type hypersensitivity to leishmanin in resistance to Leishmania major–associated cutaneous leishmaniasis. J. Inf. Dis. 192, 1981–1987. 10.1086/498042 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

