Armc8 is an evolutionarily conserved armadillo protein involved in cell-cell adhesion complexes through multiple molecular interactions
- PMID: 30482882
- PMCID: PMC6680376
- DOI: 10.1042/BSR20180604
Armc8 is an evolutionarily conserved armadillo protein involved in cell-cell adhesion complexes through multiple molecular interactions
Abstract
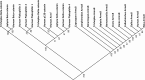
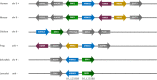
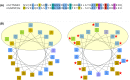
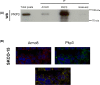
Armadillo-repeat-containing protein 8 (Armc8) belongs to the family of armadillo-repeat containing proteins, which have been found to be involved in diverse cellular functions including cell-cell contacts and intracellular signaling. By comparative analyses of armadillo repeat protein structures and genomes from various premetazoan and metazoan species, we identified orthologs of human Armc8 and analyzed in detail the evolutionary relationship of Armc8 genes and their encoded proteins. Armc8 is a highly ancestral armadillo protein although not present in yeast. Consequently, Armc8 is not the human ortholog of yeast Gid5/Vid28.Further, we performed a candidate approach to characterize new protein interactors of Armc8. Interactions between Armc8 and specific δ-catenins (plakophilins-1, -2, -3 and p0071) were observed by the yeast two-hybrid approach and confirmed by co-immunoprecipitation and co-localization. We also showed that Armc8 interacts specifically with αE-catenin but neither with αN-catenin nor with αT-catenin. Degradation of αE-catenin has been reported to be important in cancer and to be regulated by Armc8. A similar process may occur with respect to plakophilins in desmosomes. Deregulation of desmosomal proteins has been considered to contribute to tumorigenesis.
Keywords: Armadillo protein; Armc8; desmosome; molecular evolution.
© 2019 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


Similar articles
-
Proteasome-dependent degradation of alpha-catenin is regulated by interaction with ARMc8alpha.Biochem J. 2008 May 1;411(3):581-91. doi: 10.1042/BJ20071312. Biochem J. 2008. PMID: 18215130
-
Plakophilins: Multifunctional proteins or just regulators of desmosomal adhesion?Biochim Biophys Acta. 2007 Jan;1773(1):69-77. doi: 10.1016/j.bbamcr.2006.04.009. Epub 2006 May 6. Biochim Biophys Acta. 2007. PMID: 16765467 Review.
-
Targeting of p0071 to desmosomes and adherens junctions is mediated by different protein domains.J Cell Sci. 2003 Apr 1;116(Pt 7):1219-33. doi: 10.1242/jcs.00275. J Cell Sci. 2003. PMID: 12615965
-
PAPIN. A novel multiple PSD-95/Dlg-A/ZO-1 protein interacting with neural plakophilin-related armadillo repeat protein/delta-catenin and p0071.J Biol Chem. 2000 Sep 22;275(38):29875-80. doi: 10.1074/jbc.M005384200. J Biol Chem. 2000. PMID: 10896674
-
Plakophilins in desmosomal adhesion and signaling.Cell Commun Adhes. 2014 Feb;21(1):25-42. doi: 10.3109/15419061.2013.876017. Cell Commun Adhes. 2014. PMID: 24460199 Review.
Cited by
-
The CTLH Complex in Cancer Cell Plasticity.J Oncol. 2019 Nov 30;2019:4216750. doi: 10.1155/2019/4216750. eCollection 2019. J Oncol. 2019. PMID: 31885576 Free PMC article. Review.
-
Structural and Functional Insights into GID/CTLH E3 Ligase Complexes.Int J Mol Sci. 2022 May 24;23(11):5863. doi: 10.3390/ijms23115863. Int J Mol Sci. 2022. PMID: 35682545 Free PMC article. Review.
-
ARMC Subfamily: Structures, Functions, Evolutions, Interactions, and Diseases.Front Mol Biosci. 2021 Nov 29;8:791597. doi: 10.3389/fmolb.2021.791597. eCollection 2021. Front Mol Biosci. 2021. PMID: 34912852 Free PMC article. Review.
-
Identification of a Goat Intersexuality-Associated Novel Variant Through Genome-Wide Resequencing and Hi-C.Front Genet. 2021 Feb 9;11:616743. doi: 10.3389/fgene.2020.616743. eCollection 2020. Front Genet. 2021. PMID: 33633772 Free PMC article.
-
MiR-664 Protects Against UVB Radiation-Induced HaCaT Cell Damage via Downregulating ARMC8.Dose Response. 2020 Jun 3;18(2):1559325820929234. doi: 10.1177/1559325820929234. eCollection 2020 Apr-Jun. Dose Response. 2020. PMID: 32547335 Free PMC article.
References
-
- Giardina B.J., Dunton D. and Chiang H.L. (2013) Vid28 protein is required for the association of vacuole import and degradation (Vid) vesicles with actin patches and the retention of Vid vesicle proteins in the intracellular fraction. J. Biol. Chem. 288, 11636–1164810.1074/jbc.M112.419895 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

