Merkel cell polyomavirus-specific immune responses in patients with Merkel cell carcinoma receiving anti-PD-1 therapy
- PMID: 30482247
- PMCID: PMC6258401
- DOI: 10.1186/s40425-018-0450-7
Merkel cell polyomavirus-specific immune responses in patients with Merkel cell carcinoma receiving anti-PD-1 therapy
Abstract
Background: Merkel cell carcinoma (MCC) is an aggressive skin cancer that frequently responds to anti-PD-1 therapy. MCC is associated with sun exposure and, in 80% of cases, Merkel cell polyomavirus (MCPyV). MCPyV-specific T and B cell responses provide a unique opportunity to study cancer-specific immunity throughout PD-1 blockade therapy.
Methods: Immune responses were assessed in patients (n = 26) with advanced MCC receiving pembrolizumab. Peripheral blood mononuclear cells (PBMC) were collected at baseline and throughout treatment. MCPyV-oncoprotein antibodies were quantified and T cells were assessed for MCPyV-specificity via tetramer staining and/or cytokine secretion. Pre-treatment tumor biopsies were analyzed for T cell receptor clonality.
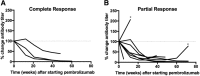
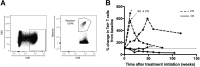
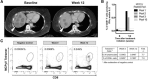
Results: MCPyV oncoprotein antibodies were detectable in 15 of 17 (88%) of virus-positive MCC (VP-MCC) patients. Antibodies decreased in 10 of 11 (91%) patients with responding tumors. Virus-specific T cells decreased over time in patients who had a complete response, and increased in patients who had persistent disease. Tumors that were MCPyV(+) had a strikingly more clonal (less diverse) intratumoral TCR repertoire than virus-negative tumors (p = 0.0001).
Conclusions: Cancer-specific T and B cell responses generally track with disease burden during PD-1 blockade, in proportion to presence of antigen. Intratumoral TCR clonality was significantly greater in VP-MCC than VN-MCC tumors, suggesting expansion of a limited number of dominant clones in response to fewer immunogenic MCPyV antigens. In contrast, VN-MCC tumors had lower clonality, suggesting a diverse T cell response to numerous neoantigens. These findings reveal differences in tumor-specific immunity for VP-MCC and VN-MCC, both of which often respond to anti-PD-1 therapy.
Keywords: Anti-PD-1; Immunotherapy; Merkel cell carcinoma; Merkel cell polyomavirus; Pembrolizumab; T cell; Viral cancer antigen.
Conflict of interest statement
Ethics approval and consent to participate
The protocol was approved by the institutional review board at each participating center, and the study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. All the patients provided written informed consent before study entry.
Consent for publication
Patients provided informed consent for publication of de-identified data. The consent is held by the Cancer Immunotherapy Trials Network and is available for review.
Competing interests
PN serves as a paid consultant for EMD Serono. Bristol Myers Squibb has provided research support to PN’s institution. SLT has research grants from Bristol-Myers Squibb, and receives consulting fees and stock from Five Prime Therapeutics; her spouse receives consulting fees from Amgen, Compugen, MedImmune, Merck, Pfizer, and Potenza Therapeutics, and receives stock options from Compugen, Jounce Therapeutics, and Potenza.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Quality Is King: Fundamental Insights into Tumor Antigenicity from Virus-Associated Merkel Cell Carcinoma.J Invest Dermatol. 2021 Aug;141(8):1897-1905. doi: 10.1016/j.jid.2020.12.037. Epub 2021 Apr 13. J Invest Dermatol. 2021. PMID: 33863500 Free PMC article. Review.
-
Merkel Cell Carcinoma in the Age of Immunotherapy: Facts and Hopes.Clin Cancer Res. 2018 May 1;24(9):2035-2043. doi: 10.1158/1078-0432.CCR-17-0439. Epub 2017 Dec 7. Clin Cancer Res. 2018. PMID: 29217527 Free PMC article. Review.
-
Robust Production of Merkel Cell Polyomavirus Oncogene Specific T Cells From Healthy Donors for Adoptive Transfer.Front Immunol. 2020 Dec 9;11:592721. doi: 10.3389/fimmu.2020.592721. eCollection 2020. Front Immunol. 2020. PMID: 33362774 Free PMC article.
-
Merkel cell polyomavirus-specific CD8⁺ and CD4⁺ T-cell responses identified in Merkel cell carcinomas and blood.Clin Cancer Res. 2011 Nov 1;17(21):6671-80. doi: 10.1158/1078-0432.CCR-11-1513. Epub 2011 Sep 9. Clin Cancer Res. 2011. PMID: 21908576 Free PMC article.
-
Tumor-Infiltrating Merkel Cell Polyomavirus-Specific T Cells Are Diverse and Associated with Improved Patient Survival.Cancer Immunol Res. 2017 Feb;5(2):137-147. doi: 10.1158/2326-6066.CIR-16-0210. Epub 2017 Jan 16. Cancer Immunol Res. 2017. PMID: 28093446 Free PMC article.
Cited by
-
Recent advances in Merkel cell carcinoma.F1000Res. 2019 Nov 26;8:F1000 Faculty Rev-1995. doi: 10.12688/f1000research.20747.1. eCollection 2019. F1000Res. 2019. PMID: 31824653 Free PMC article. Review.
-
Merkel cell polyomavirus-specific and CD39+CLA+ CD8 T cells as blood-based predictive biomarkers for PD-1 blockade in Merkel cell carcinoma.Cell Rep Med. 2024 Feb 20;5(2):101390. doi: 10.1016/j.xcrm.2023.101390. Epub 2024 Feb 9. Cell Rep Med. 2024. PMID: 38340724 Free PMC article.
-
Neoadjuvant Approaches to Non-Melanoma Skin Cancer.Cancers (Basel). 2023 Nov 21;15(23):5494. doi: 10.3390/cancers15235494. Cancers (Basel). 2023. PMID: 38067198 Free PMC article. Review.
-
Quality Is King: Fundamental Insights into Tumor Antigenicity from Virus-Associated Merkel Cell Carcinoma.J Invest Dermatol. 2021 Aug;141(8):1897-1905. doi: 10.1016/j.jid.2020.12.037. Epub 2021 Apr 13. J Invest Dermatol. 2021. PMID: 33863500 Free PMC article. Review.
-
The presence of Merkel cell carcinoma polyomavirus is associated with a distinct phenotype in neoplastic Merkel cell carcinoma cells and their tissue microenvironment.PLoS One. 2020 Jul 20;15(7):e0232517. doi: 10.1371/journal.pone.0232517. eCollection 2020. PLoS One. 2020. PMID: 32687503 Free PMC article.
References
-
- Iyer JG, Afanasiev OK, McClurkan C, Paulson K, Nagase K, Jing L, et al. Merkel cell polyomavirus-specific CD8(+) and CD4(+) T-cell responses identified in Merkel cell carcinomas and blood. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(21):6671–6680. doi: 10.1158/1078-0432.CCR-11-1513. - DOI - PMC - PubMed
-
- Church C, Markarov V, Riaz N, Chan T, Choi J, Nghiem P. Merkel cell carcinoma UV-neoantigen-specific T cells in the context of PD1 checkpoint blockade. Keystone Symposia: Cancer Immunology and Immunotherapy: Taking a Place in Mainstream Oncology (C7); Whistler, BC. 2017;Poster.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
