HIF-2α, but not HIF-1α, mediates hypoxia-induced up-regulation of Flt-1 gene expression in placental trophoblasts
- PMID: 30478339
- PMCID: PMC6255857
- DOI: 10.1038/s41598-018-35745-1
HIF-2α, but not HIF-1α, mediates hypoxia-induced up-regulation of Flt-1 gene expression in placental trophoblasts
Abstract
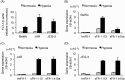
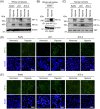
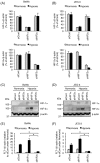
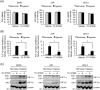
Placental hypoxia and elevated levels of circulating soluble Fms-like tyrosine kinase-1 (sFlt-1), an anti-angiogenic factor, are closely related to the pathogenesis of preeclampsia. Although sFlt-1 secretion from the placental trophoblasts is increased under hypoxic conditions, the underlying molecular mechanism remains unclear. Previously, an authentic hypoxia response element in the Flt-1 gene promoter was shown to be a potential binding site for hypoxia-inducible factors (HIFs). Here, we investigated the roles of HIF-1α and HIF-2α in Flt-1 gene expression in trophoblast-derived choriocarcinoma cell lines and cytotrophoblasts exposed to hypoxic conditions. In the cell lines, increased expression of sFlt-1 splice variants and nuclear accumulation of HIF-1α and HIF-2α were observed after hypoxic stimulation. A specific small interfering RNA or an inhibitor molecule targeting HIF-2α decreased hypoxia-induced up-regulation of Flt-1 gene expression. Moreover, in cytotrophoblasts, increased sFlt-1 mRNA expression and elevated sFlt-1 production were induced by hypoxic stimulation. Notably, hypoxia-induced elevation of sFlt-1 secretion from the cytotrophoblasts was inhibited by silencing the HIF-2α, but not HIF-1α mRNA. These findings suggest that hypoxia-induced activation of HIF-2α is essential for the increased production of sFlt-1 proteins in trophoblasts. Targeting the HIF-2α may be a novel strategy for the treatment of preeclampsia.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women.Placenta. 2005 Aug;26(7):563-73. doi: 10.1016/j.placenta.2004.09.001. Placenta. 2005. PMID: 15993706
-
[Expression of hypoxia-inducible factor-1alpha, vascular endothelial growth factor and sFlt-1 in preeclampsia placenta].Zhonghua Fu Chan Ke Za Zhi. 2006 Jul;41(7):440-4. Zhonghua Fu Chan Ke Za Zhi. 2006. PMID: 17083805 Chinese.
-
Expression of placenta growth factor, soluble fms-like tyrosine kinase-1, metal-responsive transcription factor-1, heme oxygenase 1 and hypoxia inducible factor-1α mRNAs in pre-eclampsia placenta and the effect of pre-eclampsia sera on their expression of choriocarcinoma cells.J Obstet Gynaecol Res. 2014 Oct;40(10):2095-103. doi: 10.1111/jog.12462. Epub 2014 Aug 11. J Obstet Gynaecol Res. 2014. PMID: 25132343
-
Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy.Hum Reprod Update. 2010 Jul-Aug;16(4):415-31. doi: 10.1093/humupd/dmp046. Epub 2009 Nov 19. Hum Reprod Update. 2010. PMID: 19926662 Free PMC article. Review.
-
Revisiting the HIF switch in the tumor and its immune microenvironment.Trends Cancer. 2022 Jan;8(1):28-42. doi: 10.1016/j.trecan.2021.10.004. Epub 2021 Nov 4. Trends Cancer. 2022. PMID: 34743924 Free PMC article. Review.
Cited by
-
Hypoxia and the integrated stress response promote pulmonary hypertension and preeclampsia: Implications in drug development.Drug Discov Today. 2021 Nov;26(11):2754-2773. doi: 10.1016/j.drudis.2021.07.011. Epub 2021 Jul 22. Drug Discov Today. 2021. PMID: 34302972 Free PMC article. Review.
-
Downregulation of HIF-2α Enhances Apoptosis and Limits Invasion in Human Placental JEG-3 Trophoblast Cells.Reprod Sci. 2021 Sep;28(9):2710-2717. doi: 10.1007/s43032-021-00581-8. Epub 2021 May 24. Reprod Sci. 2021. PMID: 34031851
-
Slowing of fetal growth and elevated maternal serum sFLT1:PlGF are associated with early term spontaneous labor.Am J Obstet Gynecol. 2021 Nov;225(5):520.e1-520.e10. doi: 10.1016/j.ajog.2021.04.232. Epub 2021 Apr 24. Am J Obstet Gynecol. 2021. PMID: 33901486 Free PMC article.
-
Extravillous trophoblast cell lineage development is associated with active remodeling of the chromatin landscape.Nat Commun. 2023 Aug 10;14(1):4826. doi: 10.1038/s41467-023-40424-5. Nat Commun. 2023. PMID: 37563143 Free PMC article.
-
Uteroplacental Circulation in Normal Pregnancy and Preeclampsia: Functional Adaptation and Maladaptation.Int J Mol Sci. 2021 Aug 11;22(16):8622. doi: 10.3390/ijms22168622. Int J Mol Sci. 2021. PMID: 34445328 Free PMC article. Review.
References
-
- Wang A, Rana S, Karumanchi SA. Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiology (Bethesda) 2009;24:147–158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

