Circulating Tumor and Invasive Cell Gene Expression Profile Predicts Treatment Response and Survival in Pancreatic Adenocarcinoma
- PMID: 30477242
- PMCID: PMC6315371
- DOI: 10.3390/cancers10120467
Circulating Tumor and Invasive Cell Gene Expression Profile Predicts Treatment Response and Survival in Pancreatic Adenocarcinoma
Abstract
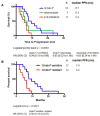
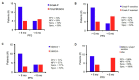
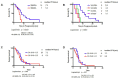
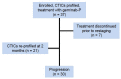
Previous studies have shown that pharmacogenomic modeling of circulating tumor and invasive cells (CTICs) can predict response of pancreatic ductal adenocarcinoma (PDAC) to combination chemotherapy, predominantly 5-fluorouracil-based. We hypothesized that a similar approach could be developed to predict treatment response to standard frontline gemcitabine with nab-paclitaxel (G/nab-P) chemotherapy. Gene expression profiles for responsiveness to G/nab-P were determined in cell lines and a test set of patient samples. A prospective clinical trial was conducted, enrolling 37 patients with advanced PDAC who received G/nab-P. Peripheral blood was collected prior to treatment, after two months of treatment, and at progression. The CTICs were isolated based on a phenotype of collagen invasion. The RNA was isolated, cDNA synthesized, and qPCR gene expression analyzed. Patients were most closely matched to one of three chemotherapy response templates. Circulating tumor and invasive cells' SMAD4 expression was measured serially. The CTICs were reliably isolated and profiled from peripheral blood prior to and during chemotherapy treatment. Individual patients could be matched to distinct response templates predicting differential responses to G/nab-P treatment. Progression free survival was significantly correlated to response prediction and ΔSMAD4 was significantly associated with disease progression. These findings support phenotypic profiling and ΔSMAD4 of CTICs as promising clinical tools for choosing effective therapy in advanced PDAC, and for anticipating disease progression.
Keywords: 5-fluorouracil; FOLFIRINOX; SMAD4; circulating tumor and invasive cells; gemcitabine; nab-paclitaxel; pancreatic cancer.
Conflict of interest statement
The following authors report no conflicts of interest: Joanne F. Chou, Marinela Capanu, Christina Covington, Maeve A. Lowery and Eileen M. O’Reilly. Kenneth H. Yu serves as an advisor to Adera Biolabs. Yu has no financial interest in and has not received compensation from Adera Biolabs. Brandon Cooper, Andrew Bartlett, Mark Ricigliano, and Brian McCarthy are employees of Adera Biolabs.
Figures
Similar articles
-
Circulating tumor and invasive cell expression profiling predicts effective therapy in pancreatic cancer.Cancer. 2022 Aug 1;128(15):2958-2966. doi: 10.1002/cncr.34269. Epub 2022 Jun 1. Cancer. 2022. PMID: 35647938 Free PMC article. Clinical Trial.
-
Pharmacogenomic modeling of circulating tumor and invasive cells for prediction of chemotherapy response and resistance in pancreatic cancer.Clin Cancer Res. 2014 Oct 15;20(20):5281-9. doi: 10.1158/1078-0432.CCR-14-0531. Epub 2014 Aug 8. Clin Cancer Res. 2014. PMID: 25107917 Free PMC article.
-
Overall survival of patients with recurrent pancreatic cancer treated with systemic therapy: a retrospective study.BMC Cancer. 2019 May 17;19(1):468. doi: 10.1186/s12885-019-5630-4. BMC Cancer. 2019. PMID: 31101022 Free PMC article.
-
Development of nanoliposomal irinotecan (nal-IRI, MM-398, PEP02) in the management of metastatic pancreatic cancer.Expert Opin Pharmacother. 2016 Jul;17(10):1413-20. doi: 10.1080/14656566.2016.1183646. Epub 2016 May 17. Expert Opin Pharmacother. 2016. PMID: 27140876 Review.
-
Spotlight on liposomal irinotecan for metastatic pancreatic cancer: patient selection and perspectives.Onco Targets Ther. 2019 Feb 21;12:1455-1463. doi: 10.2147/OTT.S167590. eCollection 2019. Onco Targets Ther. 2019. PMID: 30863113 Free PMC article. Review.
Cited by
-
Establishment and Molecular Characterization of Two Patient-Derived Pancreatic Ductal Adenocarcinoma Cell Lines as Preclinical Models for Treatment Response.Cells. 2023 Feb 11;12(4):587. doi: 10.3390/cells12040587. Cells. 2023. PMID: 36831254 Free PMC article.
-
Clinical implications and perspectives of portal venous circulating tumor cells in pancreatic cancer.World J Gastrointest Oncol. 2023 Apr 15;15(4):632-643. doi: 10.4251/wjgo.v15.i4.632. World J Gastrointest Oncol. 2023. PMID: 37123055 Free PMC article. Review.
-
Predicting Gemcitabine Delivery by 18F-FAC PET in Murine Models of Pancreatic Cancer.J Nucl Med. 2021 Feb;62(2):195-200. doi: 10.2967/jnumed.120.246926. Epub 2020 Jul 9. J Nucl Med. 2021. PMID: 32646874 Free PMC article.
-
Consequences of Mutations and Abnormal Expression of SMAD4 in Tumors and T Cells.Onco Targets Ther. 2021 Apr 13;14:2531-2540. doi: 10.2147/OTT.S297855. eCollection 2021. Onco Targets Ther. 2021. PMID: 33888990 Free PMC article. Review.
-
Analysis of capecitabine metabolites in conjunction with digital autoradiography in a murine model of pancreatic cancer suggests extensive drug penetration through the tumor.Pharmacol Res Perspect. 2022 Apr;10(2):e00898. doi: 10.1002/prp2.898. Pharmacol Res Perspect. 2022. PMID: 35257504 Free PMC article.
References
-
- Matrisian L.M., Aizenberg R., Rosenzweig A. The Alarming Rise of Pancreatic Cancer Deaths in the United States: Why We Need to Stem the Tide Today. [(accessed on 10 September 2018)]; Pancreatic Cancer Action Network, 2012. Available online: http://www.pancan.org/section_research/reports/pdf/incidence_report_2012....
-
- Poplin E., Wasan H., Rolfe L., Raponi M., Ikdahl T., Bondarenko I., Davidenko I., Bondar V., Garin A., Boeck S., et al. Randomized, Multicenter, Phase II Study of CO-101 Versus Gemcitabine in Patients With Metastatic Pancreatic Ductal Adenocarcinoma: Including a Prospective Evaluation of the Role of hENT1 in Gemcitabine or CO-101 Sensitivity. J. Clin. Oncol. 2013;31:4453–4461. doi: 10.1200/JCO.2013.51.0826. - DOI - PubMed
-
- Lamb J., Crawford E.D., Peck D., Modell J.W., Blat I.C., Wrobel M.J., Lerner J., Brunet J.P., Subramanian A., Ross K.N., et al. The Connectivity Map: Using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313:1929–1935. doi: 10.1126/science.1132939. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

