Effects of lipopolysaccharide-induced inflammation on hypoxia and inflammatory gene expression pathways of the rat testis
- PMID: 30473791
- PMCID: PMC6238406
- DOI: 10.1186/s12610-018-0079-x
Effects of lipopolysaccharide-induced inflammation on hypoxia and inflammatory gene expression pathways of the rat testis
Abstract
Background: Bacterial infection and inflammation of the testis impairs fertility, yet an understanding of inflammatory responses of the testis is incomplete. We are interested in identifying gene pathways involved in the detection and clearance of infectious microbes in the male reproductive tract. In previous studies in our lab focused on hypoxia-responsive genes of the testis, preliminary experiments suggested that genes classically categorized as hypoxia genes are also activated during antimicrobial responses. The purpose of this study was to identify hypoxia and inflammatory gene pathways that contribute to antimicrobial protection of the testis and to consider possible cross-talk and interactions between these pathways. Inflammation was induced in Sprague-Dawley rats using P. aeruginosa or E. coli lipopolysaccharide (LPS). Levels of hypoxia-inducible factor-1 (HIF-1α) protein and nuclear factor kappa B (NF-κB) were measured, and hypoxia and inflammatory gene expression patterns in testis were analyzed by gene expression profiling using real-time quantitative PCR arrays.
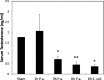
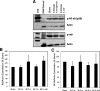
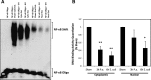
Results: In LPS-treated rats, HIF-1α protein increased with no change in Hif-1α mRNA. Western Blot analysis also demonstrated no change in NF-κB and inhibitory NFKB alpha (IκBα) protein levels following LPS treatment. Five hypoxia pathway genes (Angptl4, Egr1, Ier3, Pai1, and Glut1), and 11 inflammatory pathway genes (Ccl12, Cc13, Cd14, Cxcl10, Icam1, Il10, Il1b, Il6, Nfkbia, Tlr2, Tnf) up-regulated after 3 h of inflammation. Angptl4, Ccl12, Cc13, Cd14, Egr1, Nfkbia, Tlr2, and Tnf remained elevated at 6 h. Six genes were up-regulated at 6 h only (Bhlhe40, C3, Jak2, Nlrp3, Slc11a1, Tlr1). One gene (Tlr5) was down-regulated after 3 h and no genes at 6 h. Electrophoretic mobility shift assay results suggest a decrease in NF-κB binding activity following LPS treatment.
Conclusions: Testicular HIF-1α is up-regulated following LPS-induced inflammation. In contrast to other tissues, in which HIF-1α is up-regulated through transcriptional activation via NF-κB, we conclude that HIF-1α in the testis is not up-regulated through an increase in Hif-1α mRNA or through NF-κB-dependent mechanisms. Hypoxia pathway genes and genes involved in Toll-like receptor (TLR) and cytokine-mediated signaling comprise major functional categories of up-regulated genes, demonstrating that both hypoxia and classic inflammatory pathways are involved in inflammatory responses of the testis.
Contexte: L’infection et l’inflammation bactériennes du testicule altèrent la fertilité; cependant la compréhension des réponses inflammatoires du testicule est. encore incomplète. Nous nous sommes intéressés à l’identification des voies des gènes impliqués dans la détection et l’élimination des microbes infectieux dans l’appareil reproductif masculin. Dans de précédentes études menées dans notre laboratoire, et centrées sur des gènes sensibles à l’hypoxie, les expérimentations préliminaires suggéraient que les gènes classiquement catégorisés comme gènes de l’hypoxie étaient aussi activés au cours des réponses antimicrobiennes. Le but de la présente étude était d’identifier les voies des gènes qui contribuaient à la protection antimicrobienne du testicule et d’examiner de potentiels intermodulations et interactions entre ces voies.L’inflammation a été induite chez des rats Sprague-Dawley en utilisant des lypopolysaccharides (LPS) de P. aeruginosaet d’E. coli. Les taux de protéine du facteur-1 inductible par l’hypoxie (HIF1- α) et du facteur nucléaire kappa B (NF- kB) ont été mesurés; les profils d’expression des gènes de l’hypoxie et de l’inflammation dans le testicule ont été analysés par profilage de l’expression génique par PCR quantitative en temps réel.
Résultats: Chez les rats traités par LPS, la protéine HIF-1 α a augmenté sans modification de Hif-1αmRNA. L’analyse par Western Blot a aussi montré l’absence de modifications des taux de NF-kB et de la protéine inhibitrice NFKB alpha (IkB α) après traitement. Cinq gènes de la voie hypoxie (Angptl4, Egr1, Ier3, Pai1,et Glut1), et 11 gènes de la voie inflammatoire (Ccl12, Cc13, Cd14, Cxcl10, Icam1, Il10, Il1b, Il6, Egr1, Nfkbia, Tlr2, et Tnf) ont été régulés à la hausse après 3 heures d’inflammation. Angptl4, Ccl12, Cc13, Cd14, Egr1, Nfkbia, Tlr2, et Tnfsont restés élevés à 6 heures. Six gènes n’ont ont été régulés à la hausse qu’à 6 heures (Bhlhe40, C3, Jak2, Nlrp3, Slc11a1, Tlr1). Un gène (Tlr5) a été régulé à la baisse après 3 heures et aucun gène à 6 heures. Les résultats du test de décalage de la mobilité électrophorétique suggèrent une baisse de l’activité de liaison de NF- kB après traitement par LPS.
Conclusions: HIF-1α testiculaire est. régulé à la hausse après inflammation induite par LPS. Contrairement à d’autres tissus, dans lesquels HIF-1α est. régulé à la hausse par activation transcriptionnelle via NF- kB, nous concluons que HIF-1α dans le testicule n’est. pas régulé à la hausse par une augmentation de Hif-1 αmRNA ou par des mécanismes NF-kB-dépendants. Les gènes de la voie hypoxie et les gènes impliqués dans le récepteur Toll-like (TLR) et dans la signalisation médiée par les cytokines comprennent des catégories fonctionnelles majeures de gènes régulés à la hausse, ce qui démontre qu’à la fois les voies de l’hypoxie et les voies classiques de l’inflammation sont impliquées dans les réponses inflammatoires du testicule.
Keywords: Hypoxia; Inflammation; Lipopolysaccharide; Orchitis; Pathogens.
Conflict of interest statement
Animal research protocols were approved by the Monmouth University IACUC and conform to guidelines established in The Guide for the Care and Use of Laboratory Animals.Not applicable.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
[Hypoxia/reoxygenation and lipopolysaccharide induced nuclear factor-ΚB and hypoxia-inducible factor-1α signaling pathways in intestinal epithelial cell injury and the interventional effect of emodin].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014 Jun;26(6):409-14. doi: 10.3760/cma.j.issn.2095-4352.2014.06.009. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014. PMID: 24912640 Chinese.
-
Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-kappaB.Biochem J. 2006 Jun 15;396(3):517-27. doi: 10.1042/BJ20051839. Biochem J. 2006. PMID: 16533170 Free PMC article.
-
HIF-1α/JMJD1A signaling regulates inflammation and oxidative stress following hyperglycemia and hypoxia-induced vascular cell injury.Cell Mol Biol Lett. 2021 Sep 3;26(1):40. doi: 10.1186/s11658-021-00283-8. Cell Mol Biol Lett. 2021. PMID: 34479471 Free PMC article.
-
Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders.Cell Signal. 2013 Oct;25(10):1939-48. doi: 10.1016/j.cellsig.2013.06.007. Epub 2013 Jun 11. Cell Signal. 2013. PMID: 23770291 Review.
-
Hypoxia and Inflammation in Cancer, Focus on HIF and NF-κB.Biomedicines. 2017 May 9;5(2):21. doi: 10.3390/biomedicines5020021. Biomedicines. 2017. PMID: 28536364 Free PMC article. Review.
Cited by
-
Aging, inflammation and DNA damage in the somatic testicular niche with idiopathic germ cell aplasia.Nat Commun. 2021 Sep 1;12(1):5205. doi: 10.1038/s41467-021-25544-0. Nat Commun. 2021. PMID: 34471128 Free PMC article.
-
Effects of inflammatory and anti-inflammatory environments on the macrophage mitochondrial function.Sci Rep. 2020 Nov 23;10(1):20324. doi: 10.1038/s41598-020-77370-x. Sci Rep. 2020. PMID: 33230189 Free PMC article.
-
Understanding the Underlying Molecular Mechanisms of Meiotic Arrest during In Vitro Spermatogenesis in Rat Prepubertal Testicular Tissue.Int J Mol Sci. 2022 May 24;23(11):5893. doi: 10.3390/ijms23115893. Int J Mol Sci. 2022. PMID: 35682573 Free PMC article.
-
Differential expression of genes associated with T lymphocytes function in septic patients with hypoxemia challenge.Ann Transl Med. 2019 Dec;7(24):810. doi: 10.21037/atm.2019.12.63. Ann Transl Med. 2019. PMID: 32042826 Free PMC article.
-
Bacterial Infections Affect Male Fertility: A Focus on the Oxidative Stress-Autophagy Axis.Front Cell Dev Biol. 2021 Oct 21;9:727812. doi: 10.3389/fcell.2021.727812. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34746124 Free PMC article. Review.
References
-
- Hedger Mark P. Knobil and Neill's Physiology of Reproduction. 2015. The Immunophysiology of Male Reproduction; pp. 805–892.
-
- Ochsendorf FR. What are the consequences of sexually transmitted infections on male reproduction? Handbook of Andrology. Lawrence: Allen Press, Inc; 2009.
-
- Hamada A, Esteves SC, Agrawal A. The role of contemporary andrology in unraveling the mystery of unexplained male infertility. Open Reprod Sci J. 2011;3:27–41. doi: 10.2174/1874255601103010027. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

