NOX2 oxidase expressed in endosomes promotes cell proliferation and prostate tumour development
- PMID: 30459931
- PMCID: PMC6226044
- DOI: 10.18632/oncotarget.26237
NOX2 oxidase expressed in endosomes promotes cell proliferation and prostate tumour development
Abstract
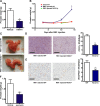
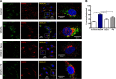
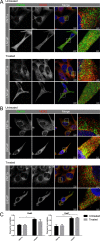
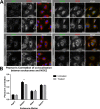
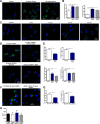
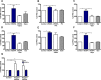
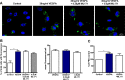
Reactive oxygen species (ROS) promote growth factor signalling including for VEGF-A and have potent angiogenic and tumourigenic properties. However, the precise enzymatic source of ROS generation, the subcellular localization of ROS production and cellular targets in vivo that influence tumour-promoting processes, are largely undefined. Here, using mRNA microarrays, we show increased gene expression for NOX2, the catalytic subunit of the ROS-generating NADPH oxidase enzyme, in human primary prostate cancer compared to non-malignant tissue. In addition, NOX4 gene expression was markedly elevated in human metastatic prostate cancers, but not in primary prostate tumours. Using a syngeneic, orthotopic mouse model of prostate cancer the genetic deletion of NOX2 (i.e. NOX2 -/y mouse) resulted in reduced angiogenesis and an almost complete failure in tumour development. Furthermore, pharmacological inhibition of NOX2 oxidase suppressed established prostate tumours in mice. In isolated endothelial cells, and in human normal and prostate cancer cells, NOX2 co-located to varying degrees with early endosome markers including EEA1, Appl1 and Rab5A and the late endosome marker Rab7A, and this correlated with significant VEGF-A-dependent ROS production within acidified endosomal compartments and endothelial cell proliferation that was NOX2 oxidase- and hydrogen peroxide dependent. We concluded that NOX2 oxidase expression and endosomal ROS production were important for prostate cancer growth and that this was required to positively regulate the VEGF pathway. The research provides a paradigm for limiting tumour growth through a better understanding of NOX2 oxidase's effect on VEGF signalling and how controlling the development of tumour vasculature can limit prostate tumour development and metastasis.
Keywords: NADPH oxidase; NOX2; endosome; prostate cancer; reactive oxygen species.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no potential conflicts of interest.
Figures

Similar articles
-
ROS-induced ROS release orchestrated by Nox4, Nox2, and mitochondria in VEGF signaling and angiogenesis.Am J Physiol Cell Physiol. 2017 Jun 1;312(6):C749-C764. doi: 10.1152/ajpcell.00346.2016. Epub 2017 Apr 19. Am J Physiol Cell Physiol. 2017. PMID: 28424170 Free PMC article.
-
NADPH oxidase isoform selective regulation of endothelial cell proliferation and survival.Naunyn Schmiedebergs Arch Pharmacol. 2009 Aug;380(2):193-204. doi: 10.1007/s00210-009-0413-0. Epub 2009 Apr 1. Naunyn Schmiedebergs Arch Pharmacol. 2009. PMID: 19337723
-
Novel endosomal NOX2 oxidase inhibitor ameliorates pandemic influenza A virus-induced lung inflammation in mice.Respirology. 2019 Oct;24(10):1011-1017. doi: 10.1111/resp.13524. Epub 2019 Mar 18. Respirology. 2019. PMID: 30884042 Free PMC article.
-
Localizing NADPH oxidase-derived ROS.Sci STKE. 2006 Aug 22;2006(349):re8. doi: 10.1126/stke.3492006re8. Sci STKE. 2006. PMID: 16926363 Review.
-
Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy.Cancer Lett. 2008 Jul 18;266(1):37-52. doi: 10.1016/j.canlet.2008.02.044. Epub 2008 Apr 10. Cancer Lett. 2008. PMID: 18406051 Free PMC article. Review.
Cited by
-
NADPH Oxidase 2 Has a Crucial Role in Cell Cycle Progression of Esophageal Squamous Cell Carcinoma.Ann Surg Oncol. 2022 Dec;29(13):8677-8687. doi: 10.1245/s10434-022-12384-5. Epub 2022 Aug 16. Ann Surg Oncol. 2022. PMID: 35972670
-
Genetically Encoded Fluorescent Redox Indicators for Unveiling Redox Signaling and Oxidative Toxicity.Chem Res Toxicol. 2021 Aug 16;34(8):1826-1845. doi: 10.1021/acs.chemrestox.1c00149. Epub 2021 Jul 20. Chem Res Toxicol. 2021. PMID: 34284580 Free PMC article. Review.
-
Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements.Biomolecules. 2019 Nov 13;9(11):735. doi: 10.3390/biom9110735. Biomolecules. 2019. PMID: 31766246 Free PMC article. Review.
-
The Role of Epac in Cancer Progression.Int J Mol Sci. 2020 Sep 5;21(18):6489. doi: 10.3390/ijms21186489. Int J Mol Sci. 2020. PMID: 32899451 Free PMC article. Review.
-
Genomic Interplay between Neoneurogenesis and Neoangiogenesis in Carcinogenesis: Therapeutic Interventions.Cancers (Basel). 2023 Mar 16;15(6):1805. doi: 10.3390/cancers15061805. Cancers (Basel). 2023. PMID: 36980690 Free PMC article. Review.
References
-
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. - PubMed
-
- Chan EC, Jiang F, Peshavariya HM, Dusting GJ. Regulation of cell proliferation by NADPH oxidase-mediated signaling: potential roles in tissue repair, regenerative medicine and tissue engineering. Pharmacol Ther. 2009;122:97–108. - PubMed
-
- Chen L, Hou X, Xiao J, Kuroda J, Ago T, Sadoshima J, Cohen RA, Tong X. Both hydrogen peroxide and transforming growth factor beta 1 contribute to endothelial Nox4 mediated angiogenesis in endothelial Nox4 transgenic mouse lines. Biochim Biophys Acta. 2014;1842:2489–2499. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

