Clustering, Spatial Distribution, and Phosphorylation of Discoidin Domain Receptors 1 and 2 in Response to Soluble Collagen I
- PMID: 30458172
- PMCID: PMC6346271
- DOI: 10.1016/j.jmb.2018.11.015
Clustering, Spatial Distribution, and Phosphorylation of Discoidin Domain Receptors 1 and 2 in Response to Soluble Collagen I
Abstract
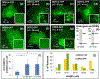
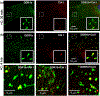
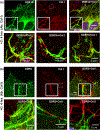
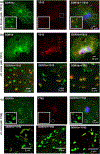
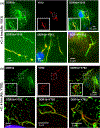
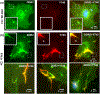
Discoidin domain receptors (DDR1 and DDR2) are receptor tyrosine kinases that signal in response to collagen. We had previously shown that collagen binding leads to clustering of DDR1b, a process partly mediated by its extracellular domain (ECD). In this study, we investigated (i) the impact of the oligomeric state of DDR2 ECD on collagen binding and fibrillogenesis, (ii) the effect of collagen binding on DDR2 clustering, and (iii) the spatial distribution and phosphorylation status of DDR1b and DDR2 after collagen stimulation. Studies were conducted using purified recombinant DDR2 ECD proteins in monomeric, dimeric or oligomeric state, and MC3T3-E1 cells expressing full-length DDR2-GFP or DDR1b-YFP. We show that the oligomeric form of DDR2 ECD displayed enhanced binding to collagen and inhibition of fibrillogenesis. Using atomic force and fluorescence microscopy, we demonstrate that unlike DDR1b, DDR2 ECD and DDR2-GFP do not undergo collagen-induced receptor clustering. However, after prolonged collagen stimulation, both DDR1b-YFP and DDR2-GFP formed filamentous structures consistent with spatial re-distribution of DDRs in cells. Immunocytochemistry revealed that while DDR1b clusters co-localized with non-fibrillar collagen, DDR1b/DDR2 filamentous structures associated with collagen fibrils. Antibodies against a tyrosine phosphorylation site in the intracellular juxtamembrane region of DDR1b displayed positive signals in both DDR1b clusters and filamentous structures. However, only the filamentous structures of both DDR1b and DDR2 co-localized with antibodies directed against tyrosine phosphorylation sites within the receptor kinase domain. Our results uncover key differences and similarities in the clustering abilities and spatial distribution of DDR1b and DDR2 and their impact on receptor phosphorylation.
Keywords: atomic force microscopy; fibrillogenesis; fluorescence microscopy; oligomer; receptor tyrosine kinase.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures
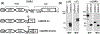




Similar articles
-
Discoidin Domain Receptors, DDR1b and DDR2, Promote Tumour Growth within Collagen but DDR1b Suppresses Experimental Lung Metastasis in HT1080 Xenografts.Sci Rep. 2020 Feb 11;10(1):2309. doi: 10.1038/s41598-020-59028-w. Sci Rep. 2020. PMID: 32047176 Free PMC article.
-
Discoidin domain receptors: Micro insights into macro assemblies.Biochim Biophys Acta Mol Cell Res. 2019 Nov;1866(11):118496. doi: 10.1016/j.bbamcr.2019.06.010. Epub 2019 Jun 21. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 31229648 Free PMC article. Review.
-
Discoidin domain receptor 2 inhibits fibrillogenesis of collagen type 1.J Mol Biol. 2006 Sep 1;361(5):864-76. doi: 10.1016/j.jmb.2006.06.067. Epub 2006 Aug 1. J Mol Biol. 2006. PMID: 16884738
-
DDR1 and DDR2 physical interaction leads to signaling interconnection but with possible distinct functions.Cell Adh Migr. 2018;12(4):324-334. doi: 10.1080/19336918.2018.1460012. Epub 2018 Jun 25. Cell Adh Migr. 2018. PMID: 29616590 Free PMC article.
-
Novel insights into the role of Discoidin domain receptor 2 (DDR2) in cancer progression: a new avenue of therapeutic intervention.Matrix Biol. 2024 Jan;125:31-39. doi: 10.1016/j.matbio.2023.12.003. Epub 2023 Dec 9. Matrix Biol. 2024. PMID: 38081526 Review.
Cited by
-
New functions of DDR1 collagen receptor in tumor dormancy, immune exclusion and therapeutic resistance.Front Oncol. 2022 Jul 22;12:956926. doi: 10.3389/fonc.2022.956926. eCollection 2022. Front Oncol. 2022. PMID: 35936735 Free PMC article. Review.
-
Collagen Kinase Receptors as Potential Therapeutic Targets in Metastatic Colon Cancer.Front Oncol. 2020 Feb 12;10:125. doi: 10.3389/fonc.2020.00125. eCollection 2020. Front Oncol. 2020. PMID: 32117772 Free PMC article. Review.
-
Synthetic peptides activating discoidin domain receptor 2 and collagen-binding integrins cooperate to stimulate osteoblast differentiation of skeletal progenitor cells.Acta Biomater. 2023 Aug;166:109-118. doi: 10.1016/j.actbio.2023.05.039. Epub 2023 May 27. Acta Biomater. 2023. PMID: 37245640 Free PMC article.
-
Two-step release of kinase autoinhibition in discoidin domain receptor 1.Proc Natl Acad Sci U S A. 2020 Sep 8;117(36):22051-22060. doi: 10.1073/pnas.2007271117. Epub 2020 Aug 24. Proc Natl Acad Sci U S A. 2020. PMID: 32839343 Free PMC article.
-
Targeting Discoidin Domain Receptors DDR1 and DDR2 overcomes matrix-mediated tumor cell adaptation and tolerance to BRAF-targeted therapy in melanoma.EMBO Mol Med. 2022 Feb 7;14(2):e11814. doi: 10.15252/emmm.201911814. Epub 2021 Dec 27. EMBO Mol Med. 2022. PMID: 34957688 Free PMC article.
References
-
- Agarwal G, DDRs and Collagen Fibrillogenesis, in: Discoidin Domain Recept. Heal. Dis, 2016: pp. 23–56.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

