In vitro Evidence That Combination Therapy With CD16-Bearing NK-92 Cells and FDA-Approved Alefacept Can Selectively Target the Latent HIV Reservoir in CD4+ CD2hi Memory T Cells
- PMID: 30455699
- PMCID: PMC6230627
- DOI: 10.3389/fimmu.2018.02552
In vitro Evidence That Combination Therapy With CD16-Bearing NK-92 Cells and FDA-Approved Alefacept Can Selectively Target the Latent HIV Reservoir in CD4+ CD2hi Memory T Cells
Abstract
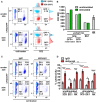
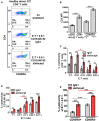
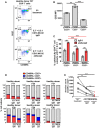
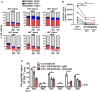
Elimination of the latent HIV reservoir remains the biggest hurdle to achieve HIV cure. In order to specifically eliminate HIV infected cells they must be distinguishable from uninfected cells. CD2 was recently identified as a potential marker enriched in the HIV-1 reservoir on CD4+ T cells, the largest, longest-lived and best-characterized constituent of the HIV reservoir. We previously proposed to repurpose FDA-approved alefacept, a humanized α-CD2 fusion protein, to reduce the HIV reservoir in CD2hi CD4+ memory T cells. Here, we show the first evidence that alefacept can specifically target and reduce CD2hi HIV infected cells in vitro. We explore a variety of natural killer (NK) cells as mediators of antibody-dependent cell-mediated cytotoxicity (ADCC) including primary NK cells, expanded NK cells as well as the CD16 transduced NK-92 cell line which is currently under study in clinical trials as a treatment for cancer. We demonstrate that CD16.NK-92 has a natural preference to kill CD2hi CD45RA- memory T cells, specifically CD45RA- CD27+ central memory/transitional memory (TCM/TM) subset in both healthy and HIV+ patient samples as well as to reduce HIV DNA from HIV+ samples from donors well controlled on antiretroviral therapy. Lastly, alefacept can combine with CD16.NK-92 to decrease HIV DNA in some patient samples and thus may yield value as part of a strategy toward sustained HIV remission.
Keywords: ADCC; CD16; CD2; FDA; HIV; NK; NK92; alefacept.
Figures


Similar articles
-
Alefacept, an immunomodulatory recombinant LFA-3/IgG1 fusion protein, induces CD16 signaling and CD2/CD16-dependent apoptosis of CD2(+) cells.J Immunol. 2002 May 1;168(9):4462-71. doi: 10.4049/jimmunol.168.9.4462. J Immunol. 2002. PMID: 11970990
-
Can We Repurpose FDA-Approved Alefacept to Diminish the HIV Reservoir?Immunotherapy (Los Angel). 2015 Dec;1(1):104. doi: 10.4172/imt.1000104. Epub 2015 Nov 30. Immunotherapy (Los Angel). 2015. PMID: 27110598 Free PMC article.
-
Innate Immune Activity Correlates with CD4 T Cell-Associated HIV-1 DNA Decline during Latency-Reversing Treatment with Panobinostat.J Virol. 2015 Oct;89(20):10176-89. doi: 10.1128/JVI.01484-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223643 Free PMC article. Clinical Trial.
-
Recent developments in the search for a cure for HIV-1 infection: targeting the latent reservoir for HIV-1.J Allergy Clin Immunol. 2014 Jul;134(1):12-9. doi: 10.1016/j.jaci.2014.05.026. Epub 2014 Jun 26. J Allergy Clin Immunol. 2014. PMID: 25117799 Review.
-
Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy.Annu Rev Immunol. 2000;18:665-708. doi: 10.1146/annurev.immunol.18.1.665. Annu Rev Immunol. 2000. PMID: 10837072 Review.
Cited by
-
Natural Killer Cell Derived Microvesicles Affect the Function of Trophoblast Cells.Membranes (Basel). 2023 Feb 9;13(2):213. doi: 10.3390/membranes13020213. Membranes (Basel). 2023. PMID: 36837716 Free PMC article.
-
Functional Assessment for Clinical Use of Serum-Free Adapted NK-92 Cells.Cancers (Basel). 2019 Jan 10;11(1):69. doi: 10.3390/cancers11010069. Cancers (Basel). 2019. PMID: 30634595 Free PMC article.
-
Development and optimization of a Zika virus antibody-dependent cell-mediated cytotoxicity (ADCC) assay.J Immunol Methods. 2021 Jan;488:112900. doi: 10.1016/j.jim.2020.112900. Epub 2020 Oct 16. J Immunol Methods. 2021. PMID: 33075363 Free PMC article.
-
Negative Regulation and Protective Function of Natural Killer Cells in HIV Infection: Two Sides of a Coin.Front Immunol. 2022 Mar 7;13:842831. doi: 10.3389/fimmu.2022.842831. eCollection 2022. Front Immunol. 2022. PMID: 35320945 Free PMC article. Review.
References
-
- Chun TW, Carruth L, Finzi D, Shen X, Digiuseppe JA, Taylor H, et al. . Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature (1997) 387:183–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

