Insulin Receptor Isoforms in Cancer
- PMID: 30453495
- PMCID: PMC6274710
- DOI: 10.3390/ijms19113615
Insulin Receptor Isoforms in Cancer
Abstract
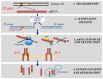
The insulin receptor (IR) mediates both metabolic and mitogenic effects especially when overexpressed or in clinical conditions with compensatory hyperinsulinemia, due to the metabolic pathway resistance, as obesity diabetes. In many cancers, IR is overexpressed preferentially as IR-A isoform, derived by alternative splicing of exon 11. The IR-A overexpression, and the increased IR-A:IR-B ratio, are mechanisms that promote the mitogenic response of cancer cells to insulin and IGF-2, which is produced locally by both epithelial and stromal cancer cells. In cancer IR-A, isoform predominance may occur for dysregulation at both mRNA transcription and post-transcription levels, including splicing factors, non-coding RNAs and protein degradation. The mechanisms that regulate IR isoform expression are complex and not fully understood. The IR isoform overexpression may play a role in cancer cell stemness, in tumor progression and in resistance to target therapies. From a clinical point of view, the IR-A overexpression in cancer may be a determinant factor for the resistance to IGF-1R target therapies for this issue. IR isoform expression in cancers may have the meaning of a predictive biomarker and co-targeting IGF-1R and IR-A may represent a new more efficacious treatment strategy.
Keywords: IGF-1R; IGF-2; cancer; hybrid receptors; insulin; insulin receptor; insulin receptor isoforms; splicing factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The Emerging Role of the Fetal Insulin Receptor in Hormone-refractory Breast Cancer.Endocrinology. 2021 Oct 1;162(10):bqab147. doi: 10.1210/endocr/bqab147. Endocrinology. 2021. PMID: 34304271 Free PMC article. Review.
-
Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease.Endocr Rev. 2009 Oct;30(6):586-623. doi: 10.1210/er.2008-0047. Epub 2009 Sep 14. Endocr Rev. 2009. PMID: 19752219 Review.
-
Insulin receptor (IR) and insulin-like growth factor receptor 1 (IGF-1R) signaling systems: novel treatment strategies for cancer.Med Oncol. 2014 Jan;31(1):805. doi: 10.1007/s12032-013-0805-3. Epub 2013 Dec 14. Med Oncol. 2014. PMID: 24338270 Review.
-
The insulin receptor isoform exon 11- (IR-A) in cancer and other diseases: a review.Horm Metab Res. 2003 Nov-Dec;35(11-12):778-85. doi: 10.1055/s-2004-814157. Horm Metab Res. 2003. PMID: 14710358 Review.
-
The role of insulin receptor isoforms and hybrid insulin/IGF-I receptors in human cancer.Curr Pharm Des. 2007;13(7):671-86. doi: 10.2174/138161207780249173. Curr Pharm Des. 2007. PMID: 17346183 Review.
Cited by
-
Lipoic acid decreases breast cancer cell proliferation by inhibiting IGF-1R via furin downregulation.Br J Cancer. 2020 Mar;122(6):885-894. doi: 10.1038/s41416-020-0729-6. Epub 2020 Jan 28. Br J Cancer. 2020. PMID: 31988347 Free PMC article.
-
Glucose-dependent effect of insulin receptor isoforms on tamoxifen antitumor activity in estrogen receptor-positive breast cancer cells.Front Endocrinol (Lausanne). 2023 Jun 9;14:1081831. doi: 10.3389/fendo.2023.1081831. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37361518 Free PMC article.
-
Decoding the Role of Insulin-like Growth Factor 1 and Its Isoforms in Breast Cancer.Int J Mol Sci. 2024 Aug 27;25(17):9302. doi: 10.3390/ijms25179302. Int J Mol Sci. 2024. PMID: 39273251 Free PMC article. Review.
-
Insulin promotes growth in breast cancer cells through the type I IGF receptor in insulin receptor deficient cells.Exp Cell Res. 2024 Jan 1;434(1):113862. doi: 10.1016/j.yexcr.2023.113862. Epub 2023 Nov 28. Exp Cell Res. 2024. PMID: 38036052 Free PMC article.
-
Insulin Receptor in Pancreatic Cancer-Crown Witness in Cross Examination.Cancers (Basel). 2021 Oct 5;13(19):4988. doi: 10.3390/cancers13194988. Cancers (Basel). 2021. PMID: 34638472 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

