Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial
- PMID: 30449625
- PMCID: PMC6541928
- DOI: 10.1016/S0140-6736(18)32779-X
Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial
Erratum in
-
Department of Error.Lancet. 2020 Mar 7;395(10226):784. doi: 10.1016/S0140-6736(20)30454-2. Lancet. 2020. PMID: 32145794 No abstract available.
Abstract
Background: Patients with human papillomavirus (HPV)-positive oropharyngeal squamous cell carcinoma have high survival when treated with radiotherapy plus cisplatin. Whether replacement of cisplatin with cetuximab-an antibody against the epidermal growth factor receptor-can preserve high survival and reduce treatment toxicity is unknown. We investigated whether cetuximab would maintain a high proportion of patient survival and reduce acute and late toxicity.
Methods: RTOG 1016 was a randomised, multicentre, non-inferiority trial at 182 health-care centres in the USA and Canada. Eligibility criteria included histologically confirmed HPV-positive oropharyngeal carcinoma; American Joint Committee on Cancer 7th edition clinical categories T1-T2, N2a-N3 M0 or T3-T4, N0-N3 M0; Zubrod performance status 0 or 1; age at least 18 years; and adequate bone marrow, hepatic, and renal function. We randomly assigned patients (1:1) to receive either radiotherapy plus cetuximab or radiotherapy plus cisplatin. Randomisation was balanced by using randomly permuted blocks, and patients were stratified by T category (T1-T2 vs T3-T4), N category (N0-N2a vs N2b-N3), Zubrod performance status (0 vs 1), and tobacco smoking history (≤10 pack-years vs >10 pack-years). Patients were assigned to receive either intravenous cetuximab at a loading dose of 400 mg/m2 5-7 days before radiotherapy initiation, followed by cetuximab 250 mg/m2 weekly for seven doses (total 2150 mg/m2), or cisplatin 100 mg/m2 on days 1 and 22 of radiotherapy (total 200 mg/m2). All patients received accelerated intensity-modulated radiotherapy delivered at 70 Gy in 35 fractions over 6 weeks at six fractions per week (with two fractions given on one day, at least 6 h apart). The primary endpoint was overall survival, defined as time from randomisation to death from any cause, with non-inferiority margin 1·45. Primary analysis was based on the modified intention-to-treat approach, whereby all patients meeting eligibility criteria are included. This study is registered with ClinicalTrials.gov, number NCT01302834.
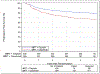
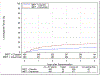
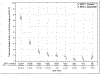
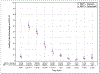
Findings: Between June 9, 2011, and July 31, 2014, 987 patients were enrolled, of whom 849 were randomly assigned to receive radiotherapy plus cetuximab (n=425) or radiotherapy plus cisplatin (n=424). 399 patients assigned to receive cetuximab and 406 patients assigned to receive cisplatin were subsequently eligible. After median follow-up duration of 4·5 years, radiotherapy plus cetuximab did not meet the non-inferiority criteria for overall survival (hazard ratio [HR] 1·45, one-sided 95% upper CI 1·94; p=0·5056 for non-inferiority; one-sided log-rank p=0·0163). Estimated 5-year overall survival was 77·9% (95% CI 73·4-82·5) in the cetuximab group versus 84·6% (80·6-88·6) in the cisplatin group. Progression-free survival was significantly lower in the cetuximab group compared with the cisplatin group (HR 1·72, 95% CI 1·29-2·29; p=0·0002; 5-year progression-free survival 67·3%, 95% CI 62·4-72·2 vs 78·4%, 73·8-83·0), and locoregional failure was significantly higher in the cetuximab group compared with the cisplatin group (HR 2·05, 95% CI 1·35-3·10; 5-year proportions 17·3%, 95% CI 13·7-21·4 vs 9·9%, 6·9-13·6). Proportions of acute moderate to severe toxicity (77·4%, 95% CI 73·0-81·5 vs 81·7%, 77·5-85·3; p=0·1586) and late moderate to severe toxicity (16·5%, 95% CI 12·9-20·7 vs 20·4%, 16·4-24·8; p=0·1904) were similar between the cetuximab and cisplatin groups.
Interpretation: For patients with HPV-positive oropharyngeal carcinoma, radiotherapy plus cetuximab showed inferior overall survival and progression-free survival compared with radiotherapy plus cisplatin. Radiotherapy plus cisplatin is the standard of care for eligible patients with HPV-positive oropharyngeal carcinoma.
Funding: National Cancer Institute USA, Eli Lilly, and The Oral Cancer Foundation.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures



Comment in
-
De-intensified treatment in human papillomavirus-positive oropharyngeal cancer.Lancet. 2019 Jan 5;393(10166):5-7. doi: 10.1016/S0140-6736(18)32930-1. Epub 2018 Nov 15. Lancet. 2019. PMID: 30449624 No abstract available.
-
De-Escalation Strategies in HPV-Associated Oropharynx Cancer-Are we Putting the Cart Before the Horse?Int J Radiat Oncol Biol Phys. 2019 Jul 15;104(4):705-709. doi: 10.1016/j.ijrobp.2019.02.054. Int J Radiat Oncol Biol Phys. 2019. PMID: 31204653 Free PMC article. No abstract available.
-
[Radiochemotherapy of HPV-positive oropharyngeal carcinomas: Cisplatin is still to be prioritized].Bull Cancer. 2020 Mar;107(3):293-294. doi: 10.1016/j.bulcan.2020.02.002. Epub 2020 Feb 26. Bull Cancer. 2020. PMID: 32113612 French. No abstract available.
Similar articles
-
Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial.Lancet. 2019 Jan 5;393(10166):51-60. doi: 10.1016/S0140-6736(18)32752-1. Epub 2018 Nov 15. Lancet. 2019. PMID: 30449623 Free PMC article. Clinical Trial.
-
Systematic evaluation and meta-analysis of the prognosis of down-staging human papillomavirus (HPV) positive oropharyngeal squamous cell carcinoma using cetuximab combined with radiotherapy instead of cisplatin combined with radiotherapy.PeerJ. 2024 May 20;12:e17391. doi: 10.7717/peerj.17391. eCollection 2024. PeerJ. 2024. PMID: 38784388 Free PMC article.
-
Phase II Trial of De-Intensified Chemoradiotherapy for Human Papillomavirus-Associated Oropharyngeal Squamous Cell Carcinoma.J Clin Oncol. 2019 Oct 10;37(29):2661-2669. doi: 10.1200/JCO.19.01007. Epub 2019 Aug 14. J Clin Oncol. 2019. PMID: 31411949 Free PMC article. Clinical Trial.
-
Comparison of different treatments for HPV+ oropharyngeal carcinoma: a network meta-analysis.Eur Arch Otorhinolaryngol. 2023 Mar;280(3):963-971. doi: 10.1007/s00405-022-07710-2. Epub 2022 Oct 20. Eur Arch Otorhinolaryngol. 2023. PMID: 36261656 Review.
-
Treatment preferences in human papillomavirus-associated oropharyngeal cancer.Future Oncol. 2018 Oct;14(24):2521-2530. doi: 10.2217/fon-2018-0063. Epub 2018 Sep 28. Future Oncol. 2018. PMID: 30265132 Free PMC article. Review.
Cited by
-
Intrinsic radiomic expression patterns after 20 Gy demonstrate early metabolic response of oropharyngeal cancers.Med Phys. 2021 Jul;48(7):3767-3777. doi: 10.1002/mp.14926. Epub 2021 Jun 2. Med Phys. 2021. PMID: 33959972 Free PMC article.
-
Quality of life following treatment with intra-arterial cisplatin with concurrent radiation and erlotinib for locally advanced head and neck cancer.Support Care Cancer. 2024 Jan 9;32(2):93. doi: 10.1007/s00520-023-08286-1. Support Care Cancer. 2024. PMID: 38193937 Free PMC article.
-
A phase 1, single centre, open label, escalating dose study to assess the safety, tolerability and immunogenicity of a therapeutic human papillomavirus (HPV) DNA vaccine (AMV002) for HPV-associated head and neck cancer (HNC).Cancer Immunol Immunother. 2021 Mar;70(3):743-753. doi: 10.1007/s00262-020-02720-7. Epub 2020 Sep 12. Cancer Immunol Immunother. 2021. PMID: 32918586 Free PMC article. Clinical Trial.
-
Role of Mesenchymal Stem/Stromal Cells in Head and Neck Cancer-Regulatory Mechanisms of Tumorigenic and Immune Activity, Chemotherapy Resistance, and Therapeutic Benefits of Stromal Cell-Based Pharmacological Strategies.Cells. 2024 Jul 28;13(15):1270. doi: 10.3390/cells13151270. Cells. 2024. PMID: 39120301 Free PMC article. Review.
-
The 3 Bs of cancer care amid the COVID-19 pandemic crisis: "Be safe, be smart, be kind"-A multidisciplinary approach increasing the use of radiation and embracing telemedicine for head and neck cancer.Cancer. 2020 Sep 15;126(18):4092-4104. doi: 10.1002/cncr.33031. Epub 2020 Jul 8. Cancer. 2020. PMID: 32639615 Free PMC article.
References
-
- Pignon JP, le Maitre A, Maillard E, Bourhis J, Group M-NC. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009;92:4–14. - PubMed
-
- Blanchard P, Baujat B, Holostenco V, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): a comprehensive analysis by tumour site. Radiother Oncol 2011;100:33–40. - PubMed
-
- Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003;21:92–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

