Oral polio vaccine response in the MAL-ED birth cohort study: Considerations for polio eradication strategies
- PMID: 30442479
- PMCID: PMC6325791
- DOI: 10.1016/j.vaccine.2018.05.080
Oral polio vaccine response in the MAL-ED birth cohort study: Considerations for polio eradication strategies
Abstract
Background: Immunization programs have leveraged decades of research to maximize oral polio vaccine (OPV) response. Moving toward global poliovirus eradication, the WHO recommended phased OPV-to-IPV replacement on schedules in 2012. Using the MAL-ED prospective birth cohort data, we evaluated the influence of early life exposures impacting OPV immunization by measuring OPV response for serotypes 1 and 3.
Methods: Polio neutralizing antibody assays were conducted at 7 and 15 months of age for serotypes 1 and 3. Analyses were conducted on children receiving ≥3 OPV doses (n = 1449). History of vaccination, feeding patterns, physical growth, home environment, diarrhea, enteropathogen detection, and gut inflammation were examined as risk factors for non-response [Log2(titer) < 3] and Log2(titer) by serotype using multivariate regression.
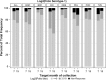
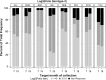
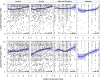
Findings: Serotype 1 seroconversion was significantly higher than serotype 3 (96.6% vs. 89.6%, 15 months). Model results indicate serotypes 1 and 3 failure was minimized following four and six OPV doses, respectively; however, enteropathogen detection and poor socioeconomic conditions attenuated response in both serotypes. At three months of age, bacterial detection in stool reduced serotype 1 and 3 Log2 titers by 0.34 (95% CI 0.14-0.54) and 0.53 (95% CI 0.29-0.77), respectively, and increased odds of serotype 3 failure by 3.0 (95% CI 1.6-5.8). Our socioeconomic index, consisting of Water, Assets, Maternal education, and Income (WAMI), was associated with a 0.79 (95% CI 0.15-1.43) and 1.23 (95% CI 0.34-2.12) higher serotype 1 and 3 Log2 titer, respectively, and a 0.04 (95% CI 0.002-0.40) lower odds of serotype 3 failure. Introduction of solids, transferrin receptor, and underweight were differentially associated with serotype response. Other factors, including diarrheal frequency and breastfeeding practices, were not associated with OPV response.
Interpretation: Under real-world conditions, improved vaccination coverage and socio-environmental conditions, and reducing early life bacterial exposures are key to improving OPV response and should inform polio eradication strategies.
Keywords: Enteropathogen infection; Home environment; Oral polio vaccination; Poliomyelitis.
Copyright © 2018 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures




Similar articles
-
Sequential inactivated (IPV) and live oral (OPV) poliovirus vaccines for preventing poliomyelitis.Cochrane Database Syst Rev. 2019 Dec 5;12(12):CD011260. doi: 10.1002/14651858.CD011260.pub2. Cochrane Database Syst Rev. 2019. PMID: 31801180 Free PMC article.
-
Inactivated poliovirus vaccine given alone or in a sequential schedule with bivalent oral poliovirus vaccine in Chilean infants: a randomised, controlled, open-label, phase 4, non-inferiority study.Lancet Infect Dis. 2015 Nov;15(11):1273-82. doi: 10.1016/S1473-3099(15)00219-4. Epub 2015 Aug 26. Lancet Infect Dis. 2015. PMID: 26318714 Clinical Trial.
-
Vaccine schedules and the effect on humoral and intestinal immunity against poliovirus: a systematic review and network meta-analysis.Lancet Infect Dis. 2019 Oct;19(10):1121-1128. doi: 10.1016/S1473-3099(19)30301-9. Epub 2019 Jul 23. Lancet Infect Dis. 2019. PMID: 31350192
-
Poliovirus vaccines. Progress toward global poliomyelitis eradication and changing routine immunization recommendations in the United States.Pediatr Clin North Am. 2000 Apr;47(2):287-308. doi: 10.1016/s0031-3955(05)70208-x. Pediatr Clin North Am. 2000. PMID: 10761505 Review.
-
Polio endgame: the global introduction of inactivated polio vaccine.Expert Rev Vaccines. 2015 May;14(5):749-62. doi: 10.1586/14760584.2015.1001750. Epub 2015 Jan 19. Expert Rev Vaccines. 2015. PMID: 25597843 Review.
Cited by
-
Effects of Iron Status on Adaptive Immunity and Vaccine Efficacy: A Review.Adv Nutr. 2024 Jun;15(6):100238. doi: 10.1016/j.advnut.2024.100238. Epub 2024 May 8. Adv Nutr. 2024. PMID: 38729263 Free PMC article. Review.
-
Advancing sustainable development goals through immunization: a literature review.Global Health. 2021 Aug 26;17(1):95. doi: 10.1186/s12992-021-00745-w. Global Health. 2021. PMID: 34446050 Free PMC article. Review.
-
Intestinal permeability and inflammation mediate the association between nutrient density of complementary foods and biochemical measures of micronutrient status in young children: results from the MAL-ED study.Am J Clin Nutr. 2019 Oct 1;110(4):1015-1025. doi: 10.1093/ajcn/nqz151. Am J Clin Nutr. 2019. PMID: 31565748 Free PMC article.
-
Intestinal Colonization With Bifidobacterium longum Subspecies Is Associated With Length at Birth, Exclusive Breastfeeding, and Decreased Risk of Enteric Virus Infections, but Not With Histo-Blood Group Antigens, Oral Vaccine Response or Later Growth in Three Birth Cohorts.Front Pediatr. 2022 Feb 16;10:804798. doi: 10.3389/fped.2022.804798. eCollection 2022. Front Pediatr. 2022. PMID: 35252058 Free PMC article.
-
A highly immunogenic UVC inactivated Sabin based polio vaccine.NPJ Vaccines. 2024 Nov 14;9(1):217. doi: 10.1038/s41541-024-00995-w. NPJ Vaccines. 2024. PMID: 39543143 Free PMC article.
References
-
- Global Polio Eradication Initiative. Polio cases worldwide: global polio eradication initiative; 2018. Available from: <http://www.polioeradication.org/Dataandmonitoring/Poliothisweek/Polioca....
-
- PolioEradication.Org. Circulating vaccine derived poliovirus; 2017. Available from: <http://polioeradication.org/polio-today/polio-now/this-week/circulating....
-
- Sutter R.W., Platt L., Mach O., Jafari H., Aylward R.B. The new polio eradication end game: rationale and supporting evidence. J Infect Dis. 2014;210(Suppl 1):S434–S438. - PubMed
-
- Domok I. Experiences associated with the use of live poliovirus vaccine in Hungary, 1959–1982. Rev Infect Dis. 1984;6(Suppl 2):S413–S418. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

