Streptolysin O derived from Streptococcus pyogenes inhibits RANKL‑induced osteoclastogenesis through the NF‑κB signaling pathway
- PMID: 30431141
- PMCID: PMC6297742
- DOI: 10.3892/mmr.2018.9662
Streptolysin O derived from Streptococcus pyogenes inhibits RANKL‑induced osteoclastogenesis through the NF‑κB signaling pathway
Abstract
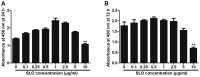
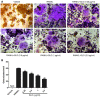
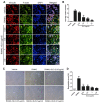
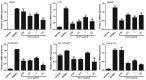
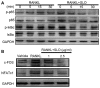
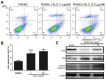
Streptococcus pyogenes (GAS) is a clinically significant bacterial strain that causes bacterial arthritis, osteomyelitis and implant infections. Infection complications can lead to serious bone destruction. Osteoclasts, the only type of cell with bone resorption function, participate in this process. Streptolysin O (SLO) is produced by almost all clinical Streptococcus pyogenes isolates. However, the role of SLO in bone infection caused by GAS had not been previously examined. The current study was performed to define the effects of SLO on receptor activator of NF‑κB ligand‑stimulated osteoclast differentiation in vitro. Results demonstrated that SLO decreased the phosphorylation of p65 and NF‑κB inhibitor α, suppressed c‑FOS and nuclear factor of activated T‑cells cytoplasmic 1, and downregulated the expression of osteoclast marker genes. SLO also induced apoptosis of mature osteoclasts. The results suggested that SLO blocked osteoclast activation during GAS infection. These findings may prove useful in the development of novel strategies for treating GAS‑associated bone infectious diseases.
Keywords: GAS; SLO; bone infection; osteoclast; NF-κB.
Figures
Similar articles
-
Schistosoma japonicum cystatin suppresses osteoclastogenesis via manipulating the NF‑κB signaling pathway.Mol Med Rep. 2021 Apr;23(4):273. doi: 10.3892/mmr.2021.11912. Epub 2021 Feb 12. Mol Med Rep. 2021. PMID: 33576450 Free PMC article.
-
α-Linolenic Acid Inhibits Receptor Activator of NF-κB Ligand Induced (RANKL-Induced) Osteoclastogenesis and Prevents Inflammatory Bone Loss via Downregulation of Nuclear Factor-KappaB-Inducible Nitric Oxide Synthases (NF-κB-iNOS) Signaling Pathways.Med Sci Monit. 2017 Oct 24;23:5056-5069. doi: 10.12659/msm.904795. Med Sci Monit. 2017. PMID: 29061958 Free PMC article.
-
Bajijiasu Abrogates Osteoclast Differentiation via the Suppression of RANKL Signaling Pathways through NF-κB and NFAT.Int J Mol Sci. 2017 Jan 19;18(1):203. doi: 10.3390/ijms18010203. Int J Mol Sci. 2017. PMID: 28106828 Free PMC article.
-
Shikimic Acid Inhibits Osteoclastogenesis in Vivo and in Vitro by Blocking RANK/TRAF6 Association and Suppressing NF-κB and MAPK Signaling Pathways.Cell Physiol Biochem. 2018;51(6):2858-2871. doi: 10.1159/000496039. Epub 2018 Dec 14. Cell Physiol Biochem. 2018. PMID: 30562759
-
Crocin inhibits RANKL‑induced osteoclastogenesis by regulating JNK and NF‑κB signaling pathways.Mol Med Rep. 2018 Jun;17(6):7947-7951. doi: 10.3892/mmr.2018.8835. Epub 2018 Mar 29. Mol Med Rep. 2018. PMID: 29620194
Cited by
-
Bacterial cholesterol-dependent cytolysins and their interaction with the human immune response.Curr Opin Infect Dis. 2024 Jun 1;37(3):164-169. doi: 10.1097/QCO.0000000000001010. Epub 2024 Mar 21. Curr Opin Infect Dis. 2024. PMID: 38527455 Review.
-
New perspectives on traumatic bone infections.Chin J Traumatol. 2020 Dec;23(6):314-318. doi: 10.1016/j.cjtee.2020.05.009. Epub 2020 Jun 2. Chin J Traumatol. 2020. PMID: 32847694 Free PMC article. Review.
References
-
- Kim J, Yang J, Park OJ, Kang SS, Kim WS, Kurokawa K, Yun CH, Kim HH, Lee BL, Han SH. Lipoproteins are an important bacterial component responsible for bone destruction through the induction of osteoclast differentiation and activation. J Bone Miner Res. 2013;28:2381–2391. doi: 10.1002/jbmr.1973. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

