Macrophages mediate corticotomy-accelerated orthodontic tooth movement
- PMID: 30429494
- PMCID: PMC6235963
- DOI: 10.1038/s41598-018-34907-5
Macrophages mediate corticotomy-accelerated orthodontic tooth movement
Abstract
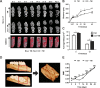
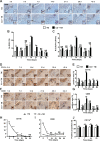
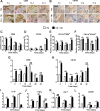
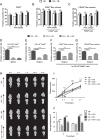
Clinical evidence has suggested that surgical corticotomy of the alveolar bone can accelerate local orthodontic tooth movement (OTM), but the underlying cell and molecular mechanisms remain largely unclear. The present study examined the role of macrophages played in corticotomy-assisted OTM. Orthodontic nickel-titanium springs were applied to the left maxillary first molars of rats or mice to induce OTM with or without corticotomy. Corticotomy enhanced OTM distance by accelerating movement through induction of local osteoclastogenesis and macrophage infiltration during OTM. Further analysis showed that macrophages were polarized toward an M1-like phenotype immediately after corticotomy and then switched to an M2-like phenotype during OTM. The microenvironment of corticotomy induced macrophage infiltration and polarization through the production of TNF-α. More importantly, the amount of OTM induced by corticotomy was significantly decreased after mice were depleted of monocyte/macrophages by injection of liposome-encapsulated clodronate. Further experiments by incubating cultured macrophages with fresh tissue suspension obtained from post-corticotomy gingiva switched the cells to an M1 phenotype through activation of the nuclear factor-κB (NF-κB) signaling pathway, and to an M2 phenotype through activation of the JAK/STAT3 signaling pathway. Our results suggest that corticotomy induces macrophage polarization first by activating the NF-κB signaling pathway and later by activating the JAK/STAT3 signaling pathway, and that these processes contribute to OTM by triggering production of inflammatory cytokines and osteoclastogenesis.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
M1-like Macrophage Polarization Promotes Orthodontic Tooth Movement.J Dent Res. 2015 Sep;94(9):1286-94. doi: 10.1177/0022034515589714. Epub 2015 Jun 29. J Dent Res. 2015. PMID: 26124217
-
Remote Corticotomy Accelerates Orthodontic Tooth Movement in a Rat Model.Biomed Res Int. 2019 Jun 17;2019:4934128. doi: 10.1155/2019/4934128. eCollection 2019. Biomed Res Int. 2019. PMID: 31317031 Free PMC article.
-
Corticotomy-assisted orthodontic enhancement by bone morphogenetic protein-2 administration.J Oral Maxillofac Surg. 2012 Feb;70(2):e124-32. doi: 10.1016/j.joms.2011.10.020. J Oral Maxillofac Surg. 2012. PMID: 22260914
-
Efficacy of surgical and non-surgical interventions on accelerating orthodontic tooth movement: a systematic review.Eur J Oral Implantol. 2015 Spring;8(1):9-24. Eur J Oral Implantol. 2015. PMID: 25738176 Review.
-
Immune Changes Induced by Orthodontic Forces: A Critical Review.J Dent Res. 2022 Jan;101(1):11-20. doi: 10.1177/00220345211016285. Epub 2021 Jun 9. J Dent Res. 2022. PMID: 34105404 Review.
Cited by
-
Mechanically induced M2 macrophages are involved in bone remodeling of the midpalatal suture during palatal expansion.Prog Orthod. 2024 Aug 5;25(1):30. doi: 10.1186/s40510-024-00529-z. Prog Orthod. 2024. PMID: 39098934 Free PMC article.
-
Consideration of hormonal changes for orthodontic treatment during pregnancy and lactation - a review.Reprod Biol Endocrinol. 2024 Aug 20;22(1):106. doi: 10.1186/s12958-024-01281-z. Reprod Biol Endocrinol. 2024. PMID: 39164703 Free PMC article. Review.
-
Osteoimmunology in Periodontitis and Orthodontic Tooth Movement.Curr Osteoporos Rep. 2023 Apr;21(2):128-146. doi: 10.1007/s11914-023-00774-x. Epub 2023 Mar 2. Curr Osteoporos Rep. 2023. PMID: 36862360 Free PMC article. Review.
-
Orthodontic tooth movement alters cementocyte ultrastructure and cellular cementum proteome signature.Bone. 2021 Dec;153:116139. doi: 10.1016/j.bone.2021.116139. Epub 2021 Aug 5. Bone. 2021. PMID: 34364013 Free PMC article.
-
Biological mechanism of surgery-mediated acceleration of orthodontic tooth movement: A narrative review.J Int Med Res. 2022 Sep;50(9):3000605221123904. doi: 10.1177/03000605221123904. J Int Med Res. 2022. PMID: 36124927 Free PMC article. Review.
References
-
- Gil A.P.S., Haas O.L., Méndez-Manjón I., Masiá-Gridilla J., Valls-Ontañón A., Hernández-Alfaro F., Guijarro-Martínez R. Alveolar corticotomies for accelerated orthodontics: A systematic review. Journal of Cranio-Maxillofacial Surgery. 2018;46(3):438–445. doi: 10.1016/j.jcms.2017.12.030. - DOI - PubMed
-
- Al Makhmari SA, Kaklamanos EG, Athanasiou AE. Short-term and long-term effectiveness of powered toothbrushes in promoting periodontal health during orthodontic treatment: A systematic review and meta-analysis. American journal of orthodontics and dentofacial orthopedics: official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics. 2017;152:753–766.e757. doi: 10.1016/j.ajodo.2017.09.003. - DOI - PubMed
-
- Lee YJ, Lee TY. External root resorption during orthodontic treatment in root-filled teeth and contralateral teeth with vital pulp: A clinical study of contributing factors. American journal of orthodontics and dentofacial orthopedics: official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics. 2016;149:84–91. doi: 10.1016/j.ajodo.2015.06.027. - DOI - PubMed
-
- Owen KM, et al. Elevation of a full-thickness mucoperiosteal flap alone accelerates orthodontic tooth movement. American journal of orthodontics and dentofacial orthopedics: official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics. 2017;152:49–57. doi: 10.1016/j.ajodo.2016.11.026. - DOI - PubMed
-
- Al-Naoum F, Hajeer MY, Al-Jundi A. Does alveolar corticotomy accelerate orthodontic tooth movement when retracting upper canines? A split-mouth design randomized controlled trial. Journal of oral and maxillofacial surgery: official journal of the American Association of Oral and Maxillofacial Surgeons. 2014;72:1880–1889. doi: 10.1016/j.joms.2014.05.003. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

