Neuraminidase 1-mediated desialylation of the mucin 1 ectodomain releases a decoy receptor that protects against Pseudomonas aeruginosa lung infection
- PMID: 30429216
- PMCID: PMC6333888
- DOI: 10.1074/jbc.RA118.006022
Neuraminidase 1-mediated desialylation of the mucin 1 ectodomain releases a decoy receptor that protects against Pseudomonas aeruginosa lung infection
Abstract
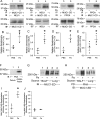
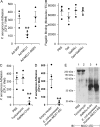
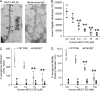
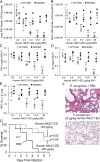
Pseudomonas aeruginosa (Pa) expresses an adhesin, flagellin, that engages the mucin 1 (MUC1) ectodomain (ED) expressed on airway epithelia, increasing association of MUC1-ED with neuraminidase 1 (NEU1) and MUC1-ED desialylation. The MUC1-ED desialylation unmasks both cryptic binding sites for Pa and a protease recognition site, permitting its proteolytic release as a hyperadhesive decoy receptor for Pa. We found here that intranasal administration of Pa strain K (PAK) to BALB/c mice increases MUC1-ED shedding into the bronchoalveolar compartment. MUC1-ED levels increased as early as 12 h, peaked at 24-48 h with a 7.8-fold increase, and decreased by 72 h. The a-type flagellin-expressing PAK strain and the b-type flagellin-expressing PAO1 strain stimulated comparable levels of MUC1-ED shedding. A flagellin-deficient PAK mutant provoked dramatically reduced MUC1-ED shedding compared with the WT strain, and purified flagellin recapitulated the WT effect. In lung tissues, Pa increased association of NEU1 and protective protein/cathepsin A with MUC1-ED in reciprocal co-immunoprecipitation assays and stimulated MUC1-ED desialylation. NEU1-selective sialidase inhibition protected against Pa-induced MUC1-ED desialylation and shedding. In Pa-challenged mice, MUC1-ED-enriched bronchoalveolar lavage fluid (BALF) inhibited flagellin binding and Pa adhesion to human airway epithelia by up to 44% and flagellin-driven motility by >30%. Finally, Pa co-administration with recombinant human MUC1-ED dramatically diminished lung and BALF bacterial burden, proinflammatory cytokine levels, and pulmonary leukostasis and increased 5-day survival from 0% to 75%. We conclude that Pa flagellin provokes NEU1-mediated airway shedding of MUC1-ED, which functions as a decoy receptor protecting against lethal Pa lung infection.
Keywords: Pseudomonas aeruginosa (P. aeruginosa); adhesin; cell surface associated (MUC1); decoy protein; flagellin; mucin 1; neuraminidase; sialic acid; sialidase; virulence.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




Similar articles
-
MUC1 ectodomain is a flagellin-targeting decoy receptor and biomarker operative during Pseudomonas aeruginosa lung infection.Sci Rep. 2021 Nov 22;11(1):22725. doi: 10.1038/s41598-021-02242-x. Sci Rep. 2021. PMID: 34811449 Free PMC article.
-
NEU1 Sialidase Regulates Membrane-tethered Mucin (MUC1) Ectodomain Adhesiveness for Pseudomonas aeruginosa and Decoy Receptor Release.J Biol Chem. 2015 Jul 24;290(30):18316-31. doi: 10.1074/jbc.M115.657114. Epub 2015 May 11. J Biol Chem. 2015. PMID: 25963144 Free PMC article.
-
Stenotrophomonas maltophilia provokes NEU1-mediated release of a flagellin-binding decoy receptor that protects against lethal infection.iScience. 2024 Aug 31;27(9):110866. doi: 10.1016/j.isci.2024.110866. eCollection 2024 Sep 20. iScience. 2024. PMID: 39314239 Free PMC article.
-
Pseudomonas aeruginosa: breaking down barriers.Curr Genet. 2016 Feb;62(1):109-13. doi: 10.1007/s00294-015-0522-x. Epub 2015 Sep 25. Curr Genet. 2016. PMID: 26407972 Free PMC article. Review.
-
Novel understandings of host cell mechanisms involved in chronic lung infection: Pseudomonas aeruginosa in the cystic fibrotic lung.J Infect Public Health. 2019 Mar-Apr;12(2):242-246. doi: 10.1016/j.jiph.2018.10.014. Epub 2018 Nov 17. J Infect Public Health. 2019. PMID: 30459101 Review.
Cited by
-
[Biofilm Eradication Four-Step Strategy: Study of Using Self-Assembled Azithromycin/Rhamnolipid Nanoparticles for Removing Pseudomonas aeruginosa Biofilm].Sichuan Da Xue Xue Bao Yi Xue Ban. 2021 Jul;52(4):598-604. doi: 10.12182/20210760207. Sichuan Da Xue Xue Bao Yi Xue Ban. 2021. PMID: 34323037 Free PMC article. Chinese.
-
Infection and the Glycome─New Insights into Host Response.ACS Infect Dis. 2024 Aug 9;10(8):2540-2550. doi: 10.1021/acsinfecdis.4c00315. Epub 2024 Jul 11. ACS Infect Dis. 2024. PMID: 38990078 Free PMC article. Review.
-
The role of sialidase Neu1 in respiratory diseases.Respir Res. 2024 Mar 19;25(1):134. doi: 10.1186/s12931-024-02763-9. Respir Res. 2024. PMID: 38500102 Free PMC article. Review.
-
Bulky glycocalyx drives cancer invasiveness by modulating substrate-specific adhesion.PNAS Nexus. 2024 Aug 22;3(8):pgae335. doi: 10.1093/pnasnexus/pgae335. eCollection 2024 Aug. PNAS Nexus. 2024. PMID: 39211517 Free PMC article.
-
MUC1 ectodomain is a flagellin-targeting decoy receptor and biomarker operative during Pseudomonas aeruginosa lung infection.Sci Rep. 2021 Nov 22;11(1):22725. doi: 10.1038/s41598-021-02242-x. Sci Rep. 2021. PMID: 34811449 Free PMC article.
References
-
- Pier G. B., and Ramphal R. (2010) Pseudomonas aeruginosa. in Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases (Mandell G. L., Bennett J. E., and Dolin R., eds), pp. 2835–2860, Elsevier/Churchill/Livingstone, Philadelphia, PA
-
- Dasgupta N., Arora S. K., and Ramphal R. (2004) The flagellar system of Pseudomonas aeruginosa. in Pseudomonas. Vol. 1 Genomics, Life Style and Molecular Architecture (Ramos J.-L., ed), pp. 675–698, Springer, Boston, MA: 10.1007/978-1-4419-9086-0 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

