Furanoid F-Acid F6 Uniquely Induces NETosis Compared to C16 and C18 Fatty Acids in Human Neutrophils
- PMID: 30428625
- PMCID: PMC6315434
- DOI: 10.3390/biom8040144
Furanoid F-Acid F6 Uniquely Induces NETosis Compared to C16 and C18 Fatty Acids in Human Neutrophils
Abstract
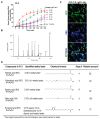
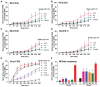
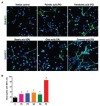
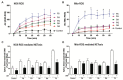
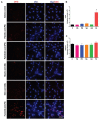
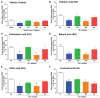
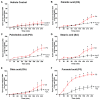
Various biomolecules induce neutrophil extracellular trap (NET) formation or NETosis. However, the effect of fatty acids on NETosis has not been clearly established. In this study, we focused on the NETosis-inducing ability of several lipid molecules. We extracted the lipid molecules present in Arabian Gulf catfish (Arius bilineatus, Val) skin gel, which has multiple therapeutic activities. Gas chromatography⁻mass spectrometry (GC-MS) analysis of the lipid fraction-3 from the gel with NETosis-inducing activity contained fatty acids including a furanoid F-acid (F6; 12,15-epoxy-13,14-dimethyleicosa-12,14-dienoic acid) and common long-chain fatty acids such as palmitic acid (PA; C16:0), palmitoleic acid (PO; C16:1), stearic acid (SA; C18:0), and oleic acid (OA; C18:1). Using pure molecules, we show that all of these fatty acids induce NETosis to different degrees in a dose-dependent fashion. Notably, F6 induces a unique form of NETosis that is rapid and induces reactive oxygen species (ROS) production by both NADPH oxidase (NOX) and mitochondria. F6 also induces citrullination of histone. By contrast, the common fatty acids (PA, PO, SA, and OA) only induce NOX-dependent NETosis. The activation of the kinases such as ERK (extracellular signal-regulated kinase) and JNK (c-Jun N-terminal kinase) is important for long-chain fatty acid-induced NETosis, whereas, in F-acid-induced NETosis, Akt is additionally needed. Nevertheless, NETosis induced by all of these compounds requires the final chromatin decondensation step of transcriptional firing. These findings are useful for understanding F-acid- and other fatty acid-induced NETosis and to establish the active ingredients with therapeutic potential for regulating diseases involving NET formation.
Keywords: MAP kinases; NADPH oxidase; NETosis; ROS; catfish lipids; citrullination of histone; furanoid F-acids (F6); long-chain fatty acids; transcription.
Conflict of interest statement
The authors declare no conflict of interest. J.M.A.-H. holds patents on the use of the Arabian Gulf catfish gel for treating various diseases. C.P.-A. holds patents on inflammatory/anti-inflammatory lipid compounds unrelated to this work. N.P. filed a patent on the use of compounds unrelated to this work to suppress NETosis.
Figures
Similar articles
-
Histone Acetylation Promotes Neutrophil Extracellular Trap Formation.Biomolecules. 2019 Jan 18;9(1):32. doi: 10.3390/biom9010032. Biomolecules. 2019. PMID: 30669408 Free PMC article.
-
Histone Deacetylase Inhibitors Dose-Dependently Switch Neutrophil Death from NETosis to Apoptosis.Biomolecules. 2019 May 11;9(5):184. doi: 10.3390/biom9050184. Biomolecules. 2019. PMID: 31083537 Free PMC article.
-
SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx.Proc Natl Acad Sci U S A. 2015 Mar 3;112(9):2817-22. doi: 10.1073/pnas.1414055112. Epub 2015 Feb 17. Proc Natl Acad Sci U S A. 2015. PMID: 25730848 Free PMC article. Clinical Trial.
-
How Do ROS Induce NETosis? Oxidative DNA Damage, DNA Repair, and Chromatin Decondensation.Biomolecules. 2024 Oct 16;14(10):1307. doi: 10.3390/biom14101307. Biomolecules. 2024. PMID: 39456240 Free PMC article. Review.
-
NETosis: Molecular Mechanisms, Role in Physiology and Pathology.Biochemistry (Mosc). 2020 Oct;85(10):1178-1190. doi: 10.1134/S0006297920100065. Biochemistry (Mosc). 2020. PMID: 33202203 Free PMC article. Review.
Cited by
-
Progression of Cystic Fibrosis Lung Disease from Childhood to Adulthood: Neutrophils, Neutrophil Extracellular Trap (NET) Formation, and NET Degradation.Genes (Basel). 2019 Feb 26;10(3):183. doi: 10.3390/genes10030183. Genes (Basel). 2019. PMID: 30813645 Free PMC article. Review.
-
The implication of neutrophil extracellular traps in nonalcoholic fatty liver disease.Front Immunol. 2023 Nov 2;14:1292679. doi: 10.3389/fimmu.2023.1292679. eCollection 2023. Front Immunol. 2023. PMID: 38022519 Free PMC article. Review.
-
Long Chain Fatty Acids as Modulators of Immune Cells Function: Contribution of FFA1 and FFA4 Receptors.Front Physiol. 2021 Jul 1;12:668330. doi: 10.3389/fphys.2021.668330. eCollection 2021. Front Physiol. 2021. PMID: 34276398 Free PMC article. Review.
-
Potential Mechanism of Dermal Wound Treatment With Preparations From the Skin Gel of Arabian Gulf Catfish: A Unique Furan Fatty Acid (F6) and Cholesta-3,5-Diene (S5) Recruit Neutrophils and Fibroblasts to Promote Wound Healing.Front Pharmacol. 2020 Jun 18;11:899. doi: 10.3389/fphar.2020.00899. eCollection 2020. Front Pharmacol. 2020. PMID: 32625093 Free PMC article.
-
Targeting neutrophil extracellular traps in severe acute pancreatitis treatment.Therap Adv Gastroenterol. 2020 Nov 24;13:1756284820974913. doi: 10.1177/1756284820974913. eCollection 2020. Therap Adv Gastroenterol. 2020. PMID: 33281940 Free PMC article. Review.
References
-
- Garcia-Romo G.S., Caielli S., Vega B., Connolly J., Allantaz F., Xu Z., Punaro M., Baisch J., Guiducci C., Coffman R.L., et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

