Detection of a Conspecific Mycovirus in Two Closely Related Native and Introduced Fungal Hosts and Evidence for Interspecific Virus Transmission
- PMID: 30428556
- PMCID: PMC6266060
- DOI: 10.3390/v10110628
Detection of a Conspecific Mycovirus in Two Closely Related Native and Introduced Fungal Hosts and Evidence for Interspecific Virus Transmission
Abstract
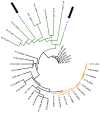
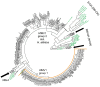
Hymenoscyphus albidus is a native fungus in Europe where it behaves as a harmless decomposer of leaves of common ash. Its close relative Hymenoscyphus fraxineus was introduced into Europe from Asia and currently threatens ash (Fraxinus sp.) stands all across the continent causing ash dieback. H. fraxineus isolates from Europe were previously shown to harbor a mycovirus named Hymenoscyphus fraxineus Mitovirus 1 (HfMV1). In the present study, we describe a conspecific mycovirus that we detected in H. albidus. HfMV1 was consistently identified in H. albidus isolates (mean prevalence: 49.3%) which were collected in the sampling areas before the arrival of ash dieback. HfMV1 strains in both fungal hosts contain a single ORF of identical length (717 AA) for which a mean pairwise identity of 94.5% was revealed. The occurrence of a conspecific mitovirus in H. albidus and H. fraxineus is most likely the result of parallel virus evolution in the two fungal hosts. HfMV1 sequences from H. albidus showed a higher nucleotide diversity and a higher number of mutations compared to those from H. fraxineus, probably due to a bottleneck caused by the introduction of H. fraxineus in Europe. Our data also points to multiple interspecific virus transfers from H. albidus to H. fraxineus, which could have contributed to the intraspecific virus diversity found in H. fraxineus.
Keywords: Chalara fraxinea; Hymenoscyphus pseudoalbidus; Narnaviridae; ash dieback; evolution; horizontal virus transmission; invasive species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Population genetic analysis of a parasitic mycovirus to infer the invasion history of its fungal host.Mol Ecol. 2017 May;26(9):2482-2497. doi: 10.1111/mec.14048. Epub 2017 Mar 9. Mol Ecol. 2017. PMID: 28160501
-
Detection and genetic characterisation of a novel mycovirus in Hymenoscyphus fraxineus, the causal agent of ash dieback.Infect Genet Evol. 2014 Dec;28:78-86. doi: 10.1016/j.meegid.2014.09.001. Epub 2014 Sep 8. Infect Genet Evol. 2014. PMID: 25219345
-
Virulence of Hymenoscyphus albidus and H. fraxineus on Fraxinus excelsior and F. pennsylvanica.PLoS One. 2015 Oct 30;10(10):e0141592. doi: 10.1371/journal.pone.0141592. eCollection 2015. PLoS One. 2015. PMID: 26517266 Free PMC article.
-
Hymenoscyphus pseudoalbidus, the causal agent of European ash dieback.Mol Plant Pathol. 2014 Jan;15(1):5-21. doi: 10.1111/mpp.12073. Epub 2013 Oct 7. Mol Plant Pathol. 2014. PMID: 24118686 Free PMC article. Review.
-
Description, Distribution, and Relevance of Viruses of the Forest Pathogen Gremmeniella abietina.Viruses. 2018 Nov 20;10(11):654. doi: 10.3390/v10110654. Viruses. 2018. PMID: 30463286 Free PMC article. Review.
Cited by
-
Towards the Forest Virome: High-Throughput Sequencing Drastically Expands Our Understanding on Virosphere in Temperate Forest Ecosystems.Microorganisms. 2021 Aug 14;9(8):1730. doi: 10.3390/microorganisms9081730. Microorganisms. 2021. PMID: 34442809 Free PMC article. Review.
-
Novel Mycoviruses Discovered from a Metatranscriptomics Survey of the Phytopathogenic Alternaria Fungus.Viruses. 2022 Nov 18;14(11):2552. doi: 10.3390/v14112552. Viruses. 2022. PMID: 36423161 Free PMC article.
-
Population Structure of Double-Stranded RNA Mycoviruses That Infect the Rice Blast Fungus Magnaporthe oryzae in Japan.Front Microbiol. 2020 Oct 28;11:593784. doi: 10.3389/fmicb.2020.593784. eCollection 2020. Front Microbiol. 2020. PMID: 33193269 Free PMC article.
-
Aspergillus Goes Viral: Ecological Insights from the Geographical Distribution of the Mycovirome within an Aspergillus flavus Population and Its Possible Correlation with Aflatoxin Biosynthesis.J Fungi (Basel). 2021 Oct 5;7(10):833. doi: 10.3390/jof7100833. J Fungi (Basel). 2021. PMID: 34682254 Free PMC article.
-
Discovery of Two Mycoviruses by High-Throughput Sequencing and Assembly of Mycovirus-Derived Small Silencing RNAs From a Hypovirulent Strain of Sclerotinia sclerotiorum.Front Microbiol. 2019 Jul 2;10:1415. doi: 10.3389/fmicb.2019.01415. eCollection 2019. Front Microbiol. 2019. PMID: 31338072 Free PMC article.
References
-
- Westphal M.I., Browne M., MacKinnon K., Noble I. The link between international trade and the global distribution of invasive alien species. Biol. Invasions. 2008;10:391–398. doi: 10.1007/s10530-007-9138-5. - DOI
-
- Santini A., Ghelardini L., De Pace C., Desprez-Loustau M.L., Capretti P., Chandelier A., Cech T., Chira D., Diamandis S., Gaitniekis T., et al. Biogeographical patterns and determinants of invasion by forest pathogens in Europe. New Phytol. 2013;197:238–250. doi: 10.1111/j.1469-8137.2012.04364.x. - DOI - PubMed
-
- Hantula J., Mu M.M., Uusivuori J. International plant trade associated risks: Laissez-faire or novel solutions. Environ. Sci. Policy. 2014;37:158–160. doi: 10.1016/j.envsci.2013.09.011. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

