Hemilabile Proton Relays and Redox Activity Lead to {FeNO} x and Significant Rate Enhancements in NO2- Reduction
- PMID: 30427681
- PMCID: PMC6668709
- DOI: 10.1021/jacs.8b08520
Hemilabile Proton Relays and Redox Activity Lead to {FeNO} x and Significant Rate Enhancements in NO2- Reduction
Abstract
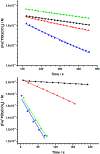
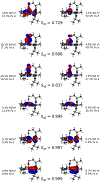
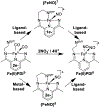
Incorporation of the triad of redox activity, hemilability, and proton responsivity into a single ligand scaffold is reported. Due to this triad, the complexes Fe(PyrrPDI)(CO)2 (3) and Fe(MorPDI)(CO)2 (4) display 40-fold enhancements in the initial rate of NO2- reduction, with respect to Fe(MeOPDI)(CO)2 (7). Utilizing the proper sterics and p Ka of the pendant base(s) to introduce hemilability into our ligand scaffolds, we report unusual {FeNO} x mononitrosyl iron complexes (MNICs) as intermediates in the NO2- reduction reaction. The {FeNO} x species behave spectroscopically and computationally similar to {FeNO}7, an unusual intermediate-spin Fe(III) coupled to triplet NO- and a singly reduced PDI ligand. These {FeNO} x MNICs facilitate enhancements in the initial rate.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
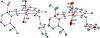

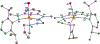
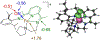
Similar articles
-
Sequential Deoxygenation of CO2 and NO2- via Redox-Control of a Pyridinediimine Ligand with a Hemilabile Phosphine.Inorg Chem. 2023 Sep 18;62(37):15173-15179. doi: 10.1021/acs.inorgchem.3c02323. Epub 2023 Sep 5. Inorg Chem. 2023. PMID: 37669231 Free PMC article.
-
Synthesis, properties, and reactivity of a series of non-heme {FeNO}(7/8) complexes: implications for Fe-nitroxyl coordination.J Inorg Biochem. 2013 Jan;118:115-27. doi: 10.1016/j.jinorgbio.2012.08.026. Epub 2012 Oct 6. J Inorg Biochem. 2013. PMID: 23116685
-
NO2(-) activation and reduction to NO by a nonheme Fe(NO2)2 complex.J Am Chem Soc. 2014 Jul 23;136(29):10230-3. doi: 10.1021/ja505236x. Epub 2014 Jul 15. J Am Chem Soc. 2014. PMID: 25010774
-
Iron(II) porphyrins induced conversion of nitrite into nitric oxide: A computational study.J Inorg Biochem. 2015 Sep;150:126-32. doi: 10.1016/j.jinorgbio.2015.06.005. Epub 2015 Jun 22. J Inorg Biochem. 2015. PMID: 26112152
-
Reaction mechanisms relevant to the formation and utilization of [Ru(edta)(NO)] complexes in aqueous media.J Inorg Biochem. 2021 Dec;225:111595. doi: 10.1016/j.jinorgbio.2021.111595. Epub 2021 Sep 3. J Inorg Biochem. 2021. PMID: 34555599 Review.
Cited by
-
Metal-Ligand Cooperative Transfer of Protons and Electrons.Trends Chem. 2021 Dec;3(12):993-996. doi: 10.1016/j.trechm.2021.10.002. Epub 2021 Oct 22. Trends Chem. 2021. PMID: 36091093 Free PMC article.
-
Biological and Bioinspired Inorganic N-N Bond-Forming Reactions.Chem Rev. 2020 Jun 24;120(12):5252-5307. doi: 10.1021/acs.chemrev.9b00629. Epub 2020 Feb 28. Chem Rev. 2020. PMID: 32108471 Free PMC article. Review.
-
Sequential Deoxygenation of CO2 and NO2- via Redox-Control of a Pyridinediimine Ligand with a Hemilabile Phosphine.Inorg Chem. 2023 Sep 18;62(37):15173-15179. doi: 10.1021/acs.inorgchem.3c02323. Epub 2023 Sep 5. Inorg Chem. 2023. PMID: 37669231 Free PMC article.
-
Harnessing the active site triad: merging hemilability, proton responsivity, and ligand-based redox-activity.Dalton Trans. 2020 Jan 28;49(4):960-965. doi: 10.1039/c9dt04470a. Epub 2020 Jan 7. Dalton Trans. 2020. PMID: 31907502 Free PMC article.
References
-
- Cosby K; Partovi KS; Crawford JH; Patel RP; Reiter CD; Martyr S; Yang BK; Waclawiw MA; Zalos G; Xu X; Huang KT; Shields H; Kim-Shapiro DB; Schechter AN; Cannon RO III; Gladwin MT Nat. Med 2003, 9, 1498–1505. - PubMed
-
- Maia LB; Moura JJ G. Chem. Rev 2014, 114, 5273–5357. - PubMed
-
- Grant SB; Saphores J-D; Feldman DL; Hamilton AJ; Fletcher TD; Cook PLM; Stewardson M; Sanders BF; Levin LA; Ambrose RF; Deletic A; Brown R; Jiang SC; Rosso D; Cooper WJ; Marusic I Science 2012, 337, 681–686. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

