Prenatal metformin exposure or organic cation transporter 3 knock-out curbs social interaction preference in male mice
- PMID: 30423430
- PMCID: PMC6388691
- DOI: 10.1016/j.phrs.2018.11.013
Prenatal metformin exposure or organic cation transporter 3 knock-out curbs social interaction preference in male mice
Abstract
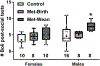
Poorly managed gestational diabetes can lead to severe complications for mother and child including fetal overgrowth, neonatal hypoglycemia and increased autism risk. Use of metformin to control it is relatively new and promising. Yet safety concerns regarding gestational metformin use remain, as its long-term effects in offspring are unclear. In light of beneficial findings with metformin for adult mouse social behavior, we hypothesized gestational metformin treatment might also promote offspring sociability. To test this, metformin was administered to non-diabetic, lean C57BL/6 J female mice at mating, with treatment discontinued at birth or wean. Male offspring exposed to metformin through birth lost social interaction preference relative to controls by time in chambers, but not by sniffing measures. Further, prenatal metformin exposure appeared to enhance social novelty preference only in females. However due to unbalanced litters and lack of statistical power, firm establishment of any sex-dependency of metformin's effects on sociability was not possible. Since organic cation transporter 3 (OCT3) transports metformin and is dense in placenta, social preferences of OCT3 knock-out males were measured. Relative to wild-type, OCT3 knock-outs had reduced interaction preference. Our data indicate gestational metformin exposure under non-diabetic conditions, or lack of OCT3, can impair social behavior in male C57BL6/J mice. Since OCT3 transports serotonin and tryptophan, impaired placental OCT3 function is one common mechanism that could persistently impact central serotonin systems and social behavior. Yet no gross alterations in serotonergic function were evident by measure of serotonin transporter density in OCT3, or serotonin turnover in metformin-exposed offspring brains. Mechanisms underlying the behavioral outcomes, and if with gestational diabetes the same would occur, remain unclear. Metformin's impacts on placental transporters and serotonin metabolism or AMPK activity in fetal brain need further investigation to clarify benefits and risks to offspring sociability from use of metformin to treat gestational diabetes.
Keywords: 1,1-dimethylbiguanide; Autism; Pregnancy; SLC22A3; Serotonin; Social behavior.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement:
None of the authors have any conflicts of interest to report.
Figures
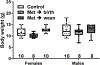



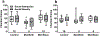


Similar articles
-
Organic Cation Transporter 3 Facilitates Fetal Exposure to Metformin during Pregnancy.Mol Pharmacol. 2018 Oct;94(4):1125-1131. doi: 10.1124/mol.118.112482. Epub 2018 Jul 16. Mol Pharmacol. 2018. PMID: 30012584 Free PMC article.
-
Metformin inhibits OCT3-mediated serotonin transport in the placenta.Biomed Pharmacother. 2024 Oct;179:117399. doi: 10.1016/j.biopha.2024.117399. Epub 2024 Sep 8. Biomed Pharmacother. 2024. PMID: 39243433
-
Taste of a pill: organic cation transporter-3 (OCT3) mediates metformin accumulation and secretion in salivary glands.J Biol Chem. 2014 Sep 26;289(39):27055-27064. doi: 10.1074/jbc.M114.570564. Epub 2014 Aug 8. J Biol Chem. 2014. PMID: 25107910 Free PMC article.
-
Interaction between Metformin, Folate and Vitamin B12 and the Potential Impact on Fetal Growth and Long-Term Metabolic Health in Diabetic Pregnancies.Int J Mol Sci. 2021 May 28;22(11):5759. doi: 10.3390/ijms22115759. Int J Mol Sci. 2021. PMID: 34071182 Free PMC article. Review.
-
The emerging role of metformin in gestational diabetes mellitus.Diabetes Obes Metab. 2017 Jun;19(6):765-772. doi: 10.1111/dom.12893. Epub 2017 Mar 24. Diabetes Obes Metab. 2017. PMID: 28127850 Review.
Cited by
-
High Affinity Decynium-22 Binding to Brain Membrane Homogenates and Reduced Dorsal Camouflaging after Acute Exposure to it in Zebrafish.Front Pharmacol. 2022 Jun 9;13:841423. doi: 10.3389/fphar.2022.841423. eCollection 2022. Front Pharmacol. 2022. PMID: 35754508 Free PMC article.
-
Organic Cation Transporters in Psychiatric Disorders.Handb Exp Pharmacol. 2021;266:215-239. doi: 10.1007/164_2021_473. Handb Exp Pharmacol. 2021. PMID: 34282486 Free PMC article.
-
Serotonin homeostasis in the materno-foetal interface at term: Role of transporters (SERT/SLC6A4 and OCT3/SLC22A3) and monoamine oxidase A (MAO-A) in uptake and degradation of serotonin by human and rat term placenta.Acta Physiol (Oxf). 2020 Aug;229(4):e13478. doi: 10.1111/apha.13478. Epub 2020 May 18. Acta Physiol (Oxf). 2020. PMID: 32311818 Free PMC article.
-
Metformin Alleviates Autistic-Like Behaviors Elicited by High-Fat Diet Consumption and Modulates the Crosstalk Between Serotonin and Gut Microbiota in Mice.Behav Neurol. 2022 Feb 17;2022:6711160. doi: 10.1155/2022/6711160. eCollection 2022. Behav Neurol. 2022. PMID: 35222739 Free PMC article.
-
Under or Absent Reporting of Light Stimuli in Testing of Anxiety-Like Behaviors in Rodents: The Need for Standardization.Front Mol Neurosci. 2022 Aug 17;15:912146. doi: 10.3389/fnmol.2022.912146. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36061362 Free PMC article.
References
-
- Xiang AH, Wang X, Martinez MP, Walthall JC, Curry ES, Page K, Buchanan TA, Coleman KJ, Getahun D, Association of maternal diabetes with autism in offspring. JAMA. 313 (2015) 1425–34. - PubMed
-
- Rowan JA, Hague WM, Gao W, Battin MR, Moore MP; MiG Trial Investigators, Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 358 (2008) 2003–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

