NF-κB Signaling in Targeting Tumor Cells by Oncolytic Viruses-Therapeutic Perspectives
- PMID: 30413032
- PMCID: PMC6265863
- DOI: 10.3390/cancers10110426
NF-κB Signaling in Targeting Tumor Cells by Oncolytic Viruses-Therapeutic Perspectives
Abstract
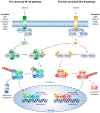
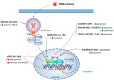
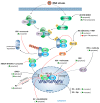
In recent years, oncolytic virotherapy became a promising therapeutic approach, leading to the introduction of a novel generation of anticancer drugs. However, despite evoking an antitumor response, introducing an oncolytic virus (OV) to the patient is still inefficient to overcome both tumor protective mechanisms and the limitation of viral replication by the host. In cancer treatment, nuclear factor (NF)-κB has been extensively studied among important therapeutic targets. The pleiotropic nature of NF-κB transcription factor includes its involvement in immunity and tumorigenesis. Therefore, in many types of cancer, aberrant activation of NF-κB can be observed. At the same time, the activity of NF-κB can be modified by OVs, which trigger an immune response and modulate NF-κB signaling. Due to the limitation of a monotherapy exploiting OVs only, the antitumor effect can be enhanced by combining OV with NF-κB-modulating drugs. This review describes the influence of OVs on NF-κB activation in tumor cells showing NF-κB signaling as an important aspect, which should be taken into consideration when targeting tumor cells by OVs.
Keywords: NF-κB signaling; immunotherapy; oncolytic viruses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Arming oncolytic viruses to leverage antitumor immunity.Expert Opin Biol Ther. 2015 Jul;15(7):959-71. doi: 10.1517/14712598.2015.1044433. Epub 2015 May 10. Expert Opin Biol Ther. 2015. PMID: 25959450 Review.
-
Recent advances in oncolytic virus-based cancer therapy.Virus Res. 2019 Sep;270:197675. doi: 10.1016/j.virusres.2019.197675. Epub 2019 Jul 25. Virus Res. 2019. PMID: 31351879 Review.
-
Immune System, Friend or Foe of Oncolytic Virotherapy?Front Oncol. 2017 May 23;7:106. doi: 10.3389/fonc.2017.00106. eCollection 2017. Front Oncol. 2017. PMID: 28589085 Free PMC article. Review.
-
Current landscape and perspective of oncolytic viruses and their combination therapies.Transl Oncol. 2022 Nov;25:101530. doi: 10.1016/j.tranon.2022.101530. Epub 2022 Sep 9. Transl Oncol. 2022. PMID: 36095879 Free PMC article. Review.
-
Dimethyl fumarate potentiates oncolytic virotherapy through NF-κB inhibition.Sci Transl Med. 2018 Jan 24;10(425):eaao1613. doi: 10.1126/scitranslmed.aao1613. Sci Transl Med. 2018. PMID: 29367345
Cited by
-
Roles of Inflammasomes in Epstein-Barr Virus-Associated Nasopharyngeal Cancer.Cancers (Basel). 2021 Apr 8;13(8):1786. doi: 10.3390/cancers13081786. Cancers (Basel). 2021. PMID: 33918087 Free PMC article. Review.
-
An overview of the potential anticancer properties of cardamonin.Explor Target Antitumor Ther. 2020;1(6):413-426. doi: 10.37349/etat.2020.00026. Epub 2020 Dec 28. Explor Target Antitumor Ther. 2020. PMID: 36046386 Free PMC article. Review.
-
Repurposing of known drugs for COVID-19 using molecular docking and simulation analysis.Bioinformation. 2023 Feb 28;19(2):149-159. doi: 10.6026/97320630019149. eCollection 2023. Bioinformation. 2023. PMID: 37814677 Free PMC article.
-
A new strategy for treating colorectal cancer: Regulating the influence of intestinal flora and oncolytic virus on interferon.Mol Ther Oncolytics. 2023 Aug 24;30:254-274. doi: 10.1016/j.omto.2023.08.010. eCollection 2023 Sep 21. Mol Ther Oncolytics. 2023. PMID: 37701850 Free PMC article. Review.
-
Endoplasmic Reticulum Stress: A Critical Molecular Driver of Endothelial Dysfunction and Cardiovascular Disturbances Associated with Diabetes.Int J Mol Sci. 2019 Apr 3;20(7):1658. doi: 10.3390/ijms20071658. Int J Mol Sci. 2019. PMID: 30987118 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

