TRIM21 Restricts Coxsackievirus B3 Replication, Cardiac and Pancreatic Injury via Interacting With MAVS and Positively Regulating IRF3-Mediated Type-I Interferon Production
- PMID: 30410495
- PMCID: PMC6209670
- DOI: 10.3389/fimmu.2018.02479
TRIM21 Restricts Coxsackievirus B3 Replication, Cardiac and Pancreatic Injury via Interacting With MAVS and Positively Regulating IRF3-Mediated Type-I Interferon Production
Abstract
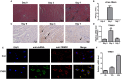
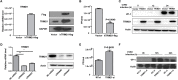
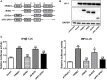
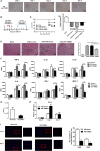
Tripartite motif-containing 21 (TRIM21) is a regulator of tissue inflammation and pro-inflammatory cytokine production, and has been implicated in negative regulation of IRF3-dependent type I interferon signaling. However, the antiviral activity of TRIM21 varies among diverse viruses and its role on regulation of type I interferon remains inconsistent in different microbial infections. Here, we investigate the potential role for TRIM21 in controlling Coxsackievirus B3 (CVB3) replication and susceptible organ pathology. We found that CVB3 infection up-regulated the expression of TRIM21 in hearts of mice and cardiomyocytes at early phase of infection. Knock-down of TRIM21 resulted in increased viral replication, while overexpression led to increased phosphorylation and dimerization of IRF3, increased IFN-β transcription and reduced viral replication in vitro. We demonstrate that TRIM21 promotes the activation of IRF3 in CVB3-infected cells via interacting with MAVS and catalyzing the K27-linked polyubiquitination of MAVS, thereby enhancing type I interferon signaling. The RING domain of ubiquitin ligase activity and PRY-SPRY domain of TRIM21 are critical for its anti-viral effect. In vivo overexpression of TRIM21 significantly protected mice against viral myocarditis by suppressing CVB3 replication and reducing cardiac inflammatory cytokine production. While TRIM21 deficient mice exhibited a decreased IFN-β production, an increased cardiac and pancreatic CVB3 replication, and aggravated pancreatic injury as well as myocarditis during acute infection. Thus, our results demonstrate TRIM21 as a positive regulator of IFN-β signaling by targeting MAVS during CVB3 infection and suggest it as a potent host defense against CVB3 infection and viral-induced injury in hearts and pancreas.
Keywords: IFN-β; IRF3; TRIM21; coxsackievirus B3 (CVB3); viral myocarditis.
Figures


Similar articles
-
Multiple Roles of TRIM21 in Virus Infection.Int J Mol Sci. 2023 Jan 14;24(2):1683. doi: 10.3390/ijms24021683. Int J Mol Sci. 2023. PMID: 36675197 Free PMC article. Review.
-
TRIM21 Promotes Innate Immune Response to RNA Viral Infection through Lys27-Linked Polyubiquitination of MAVS.J Virol. 2018 Jun 29;92(14):e00321-18. doi: 10.1128/JVI.00321-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29743353 Free PMC article.
-
Zinc finger antiviral protein inhibits coxsackievirus B3 virus replication and protects against viral myocarditis.Antiviral Res. 2015 Nov;123:50-61. doi: 10.1016/j.antiviral.2015.09.001. Epub 2015 Sep 2. Antiviral Res. 2015. PMID: 26343012
-
Over-expression of mitochondrial antiviral signaling protein inhibits coxsackievirus B3 infection by enhancing type-I interferons production.Virol J. 2012 Dec 19;9:312. doi: 10.1186/1743-422X-9-312. Virol J. 2012. PMID: 23249700 Free PMC article.
-
A Kidnapping Story: How Coxsackievirus B3 and Its Host Cell Interact.Cell Physiol Biochem. 2019;53(1):121-140. doi: 10.33594/000000125. Cell Physiol Biochem. 2019. PMID: 31230428 Review.
Cited by
-
Porcine TRIM21 Enhances Porcine Circovirus 2 Infection and Host Immune Responses, But Inhibits Apoptosis of PCV2-Infected Cells.Viruses. 2022 Jan 15;14(1):156. doi: 10.3390/v14010156. Viruses. 2022. PMID: 35062360 Free PMC article.
-
African Swine Fever Virus MGF360-14L Negatively Regulates Type I Interferon Signaling by Targeting IRF3.Front Cell Infect Microbiol. 2022 Jan 12;11:818969. doi: 10.3389/fcimb.2021.818969. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35096660 Free PMC article.
-
Construction of miRNA-target networks using microRNA profiles of CVB3-infected HeLa cells.Sci Rep. 2019 Nov 29;9(1):17876. doi: 10.1038/s41598-019-54188-w. Sci Rep. 2019. PMID: 31784561 Free PMC article.
-
DTX3L Enhances Type I Interferon Antiviral Response by Promoting the Ubiquitination and Phosphorylation of TBK1.J Virol. 2023 Jun 29;97(6):e0068723. doi: 10.1128/jvi.00687-23. Epub 2023 May 31. J Virol. 2023. PMID: 37255478 Free PMC article.
-
Multiple Roles of TRIM21 in Virus Infection.Int J Mol Sci. 2023 Jan 14;24(2):1683. doi: 10.3390/ijms24021683. Int J Mol Sci. 2023. PMID: 36675197 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

