cAMP regulation of protein phosphatases PP1 and PP2A in brain
- PMID: 30401536
- PMCID: PMC6433392
- DOI: 10.1016/j.bbamcr.2018.09.006
cAMP regulation of protein phosphatases PP1 and PP2A in brain
Abstract
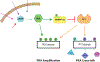
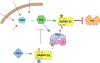
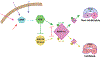
Normal functioning of the brain is dependent upon a complex web of communication between numerous cell types. Within neuronal networks, the faithful transmission of information between neurons relies on an equally complex organization of inter- and intra-cellular signaling systems that act to modulate protein activity. In particular, post-translational modifications (PTMs) are responsible for regulating protein activity in response to neurochemical signaling. The key second messenger, cyclic adenosine 3',5'-monophosphate (cAMP), regulates one of the most ubiquitous and influential PTMs, phosphorylation. While cAMP is canonically viewed as regulating the addition of phosphate groups through its activation of cAMP-dependent protein kinases, it plays an equally critical role in regulating removal of phosphate through indirect control of protein phosphatase activity. This dichotomy of regulation by cAMP places it as one of the key regulators of protein activity in response to neuronal signal transduction throughout the brain. In this review we focus on the role of cAMP in regulation of the serine/threonine phosphatases protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) and the relevance of control of PP1 and PP2A to regulation of brain function and behavior.
Keywords: Brain; PKA; PP1; PP2A; Striatum; cAMP.
Copyright © 2018. Published by Elsevier B.V.
Figures

Similar articles
-
Glucose-induced posttranslational activation of protein phosphatases PP2A and PP1 in yeast.Cell Res. 2012 Jun;22(6):1058-77. doi: 10.1038/cr.2012.20. Epub 2012 Jan 31. Cell Res. 2012. PMID: 22290422 Free PMC article.
-
Protein phosphatases 1 and 2A are both required for long-term depression and associated dephosphorylation of cAMP response element binding protein in hippocampal area CA1 in vivo.Hippocampus. 2011 Oct;21(10):1093-104. doi: 10.1002/hipo.20823. Epub 2010 Sep 7. Hippocampus. 2011. PMID: 20824729 Free PMC article.
-
Parathyroid hormone(1-34) and its analogs differentially modulate osteoblastic Rankl expression via PKA/SIK2/SIK3 and PP1/PP2A-CRTC3 signaling.J Biol Chem. 2018 Dec 28;293(52):20200-20213. doi: 10.1074/jbc.RA118.004751. Epub 2018 Oct 30. J Biol Chem. 2018. PMID: 30377251 Free PMC article.
-
Serine-threonine protein phosphatases: Lost in translation.Biochim Biophys Acta Mol Cell Res. 2019 Jan;1866(1):83-89. doi: 10.1016/j.bbamcr.2018.08.006. Epub 2018 Aug 20. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 30401537 Free PMC article. Review.
-
cAMP-regulated phosphoproteins DARPP-32, ARPP16/19, and RCS modulate striatal signal transduction through protein kinases and phosphatases.Adv Pharmacol. 2021;90:39-65. doi: 10.1016/bs.apha.2020.09.005. Epub 2020 Oct 6. Adv Pharmacol. 2021. PMID: 33706938 Review.
Cited by
-
The Cell Cycle Checkpoint System MAST(L)-ENSA/ARPP19-PP2A is Targeted by cAMP/PKA and cGMP/PKG in Anucleate Human Platelets.Cells. 2020 Feb 18;9(2):472. doi: 10.3390/cells9020472. Cells. 2020. PMID: 32085646 Free PMC article.
-
Phosphoproteomic Analysis as an Approach for Understanding Molecular Mechanisms of cAMP-Dependent Actions.Mol Pharmacol. 2021 May;99(5):342-357. doi: 10.1124/molpharm.120.000197. Epub 2021 Feb 11. Mol Pharmacol. 2021. PMID: 33574048 Free PMC article. Review.
-
The M-phase regulatory phosphatase PP2A-B55δ opposes protein kinase A on Arpp19 to initiate meiotic division.Nat Commun. 2021 Mar 23;12(1):1837. doi: 10.1038/s41467-021-22124-0. Nat Commun. 2021. PMID: 33758202 Free PMC article.
-
Characterization of a CXCR4 antagonist TIQ-15 with dual tropic HIV entry inhibition properties.PLoS Pathog. 2024 Aug 15;20(8):e1012448. doi: 10.1371/journal.ppat.1012448. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39146384 Free PMC article.
-
Chronic alcohol disrupts hypothalamic responses to stress by modifying CRF and NMDA receptor function.Neuropharmacology. 2020 May 1;167:107991. doi: 10.1016/j.neuropharm.2020.107991. Epub 2020 Feb 12. Neuropharmacology. 2020. PMID: 32059962 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

