Effect of Inorganic Nitrite vs Placebo on Exercise Capacity Among Patients With Heart Failure With Preserved Ejection Fraction: The INDIE-HFpEF Randomized Clinical Trial
- PMID: 30398602
- PMCID: PMC6248105
- DOI: 10.1001/jama.2018.14852
Effect of Inorganic Nitrite vs Placebo on Exercise Capacity Among Patients With Heart Failure With Preserved Ejection Fraction: The INDIE-HFpEF Randomized Clinical Trial
Abstract
Importance: There are few effective treatments for heart failure with preserved ejection fraction (HFpEF). Short-term administration of inorganic nitrite or nitrate preparations has been shown to enhance nitric oxide signaling, which may improve aerobic capacity in HFpEF.
Objective: To determine the effect of 4 weeks' administration of inhaled, nebulized inorganic nitrite on exercise capacity in HFpEF.
Design, setting, and participants: Multicenter, double-blind, placebo-controlled, 2-treatment, crossover trial of 105 patients with HFpEF. Participants were enrolled from July 22, 2016, to September 12, 2017, at 17 US sites, with final date of follow-up of January 2, 2018.
Interventions: Inorganic nitrite or placebo administered via micronebulizer device. During each 6-week phase of the crossover study, participants received no study drug for 2 weeks (baseline/washout) followed by study drug (nitrite or placebo) at 46 mg 3 times a day for 1 week followed by 80 mg 3 times a day for 3 weeks.
Main outcomes and measures: The primary end point was peak oxygen consumption (mL/kg/min). Secondary end points included daily activity levels assessed by accelerometry, health status as assessed by the Kansas City Cardiomyopathy Questionnaire (score range, 0-100, with higher scores reflecting better quality of life), functional class, cardiac filling pressures assessed by echocardiography, N-terminal fragment of the prohormone brain natriuretic peptide levels, other exercise indices, adverse events, and tolerability. Outcomes were assessed after treatment for 4 weeks.
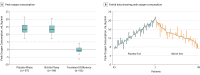
Results: Among 105 patients who were randomized (median age, 68 years; 56% women), 98 (93%) completed the trial. During the nitrite phase, there was no significant difference in mean peak oxygen consumption as compared with the placebo phase (13.5 vs 13.7 mL/kg/min; difference, -0.20 [95% CI, -0.56 to 0.16]; P = .27). There were no significant between-treatment phase differences in daily activity levels (5497 vs 5503 accelerometry units; difference, -15 [95% CI, -264 to 234]; P = .91), Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (62.6 vs 61.9; difference, 1.1 [95% CI, -1.4 to 3.5]; P = .39), functional class (2.5 vs 2.5; difference, 0.1 [95% CI, -0.1 to 0.2]; P = .43), echocardiographic E/e' ratio (16.4 vs 16.6; difference, 0.1 [95% CI, -1.2 to 1.3]; P = .93), or N-terminal fragment of the prohormone brain natriuretic peptide levels (520 vs 533 pg/mL; difference, 11 [95% CI, -53 to 75]; P = .74). Worsening heart failure occurred in 3 participants (2.9%) during the nitrite phase and 8 (7.6%) during the placebo phase.
Conclusions and relevance: Among patients with HFpEF, administration of inhaled inorganic nitrite for 4 weeks, compared with placebo, did not result in significant improvement in exercise capacity.
Trial registration: ClinicalTrials.gov Identifier: NCT02742129.
Conflict of interest statement
Figures

Comment in
- doi: 10.1001/jama.2018.16760
Similar articles
-
Effect of Praliciguat on Peak Rate of Oxygen Consumption in Patients With Heart Failure With Preserved Ejection Fraction: The CAPACITY HFpEF Randomized Clinical Trial.JAMA. 2020 Oct 20;324(15):1522-1531. doi: 10.1001/jama.2020.16641. JAMA. 2020. PMID: 33079154 Free PMC article. Clinical Trial.
-
Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial.JAMA. 2013 Mar 27;309(12):1268-77. doi: 10.1001/jama.2013.2024. JAMA. 2013. PMID: 23478662 Free PMC article. Clinical Trial.
-
Effect of Vericiguat vs Placebo on Quality of Life in Patients With Heart Failure and Preserved Ejection Fraction: The VITALITY-HFpEF Randomized Clinical Trial.JAMA. 2020 Oct 20;324(15):1512-1521. doi: 10.1001/jama.2020.15922. JAMA. 2020. PMID: 33079152 Free PMC article. Clinical Trial.
-
INDIE-HFpEF (Inorganic Nitrite Delivery to Improve Exercise Capacity in Heart Failure With Preserved Ejection Fraction): Rationale and Design.Circ Heart Fail. 2017 May;10(5):e003862. doi: 10.1161/CIRCHEARTFAILURE.117.003862. Circ Heart Fail. 2017. PMID: 28476756 Free PMC article. Review.
-
The Nitrate-Nitrite-NO Pathway and Its Implications for Heart Failure and Preserved Ejection Fraction.Curr Heart Fail Rep. 2016 Feb;13(1):47-59. doi: 10.1007/s11897-016-0277-9. Curr Heart Fail Rep. 2016. PMID: 26792295 Free PMC article. Review.
Cited by
-
Venous Tone and Stressed Blood Volume in Heart Failure: JACC Review Topic of the Week.J Am Coll Cardiol. 2022 May 10;79(18):1858-1869. doi: 10.1016/j.jacc.2022.02.050. J Am Coll Cardiol. 2022. PMID: 35512865 Free PMC article. Review.
-
Increased Risk of Heart Failure in Elderly Patients Treated with Beta-Blockers After AV Node Ablation.Am J Cardiovasc Drugs. 2023 Mar;23(2):157-164. doi: 10.1007/s40256-022-00566-1. Epub 2023 Jan 18. Am J Cardiovasc Drugs. 2023. PMID: 36652190 Free PMC article.
-
Evaluation and management of heart failure with preserved ejection fraction.Nat Rev Cardiol. 2020 Sep;17(9):559-573. doi: 10.1038/s41569-020-0363-2. Epub 2020 Mar 30. Nat Rev Cardiol. 2020. PMID: 32231333 Review.
-
Correlates of Plasma NT-proBNP/Cyclic GMP Ratio in Heart Failure With Preserved Ejection Fraction: An Analysis of the RELAX Trial.J Am Heart Assoc. 2024 Apr 2;13(7):e031796. doi: 10.1161/JAHA.123.031796. Epub 2024 Mar 27. J Am Heart Assoc. 2024. PMID: 38533961 Free PMC article. Clinical Trial.
-
Extended-Release Oral Milrinone for the Treatment of Heart Failure With Preserved Ejection Fraction.J Am Heart Assoc. 2020 Jul 7;9(13):e015026. doi: 10.1161/JAHA.119.015026. Epub 2020 Jun 18. J Am Heart Assoc. 2020. PMID: 32552264 Free PMC article. Clinical Trial.
References
-
- Redfield MM. Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2017;376(9):897. - PubMed
-
- Pieske B, Maggioni AP, Lam CSP, et al. . Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur Heart J. 2017;38(15):1119-1127. doi:10.1093/eurheartj/ehw593 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

