CD33/CD3-bispecific T-cell engaging (BiTE®) antibody construct targets monocytic AML myeloid-derived suppressor cells
- PMID: 30396365
- PMCID: PMC6217777
- DOI: 10.1186/s40425-018-0432-9
CD33/CD3-bispecific T-cell engaging (BiTE®) antibody construct targets monocytic AML myeloid-derived suppressor cells
Abstract
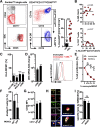
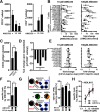
Acute myeloid leukemia (AML) is the most common acute leukemia amongst adults with a 5-year overall survival lower than 30%. Emerging evidence suggest that immune alterations favor leukemogenesis and/or AML relapse thereby negatively impacting disease outcome. Over the last years myeloid derived suppressor cells (MDSCs) have been gaining momentum in the field of cancer research. MDSCs are a heterogeneous cell population morphologically resembling either monocytes or granulocytes and sharing some key features including myeloid origin, aberrant (immature) phenotype, and immunosuppressive activity. Increasing evidence suggests that accumulating MDSCs are involved in hampering anti-tumor immune responses and immune-based therapies. Here, we demonstrate increased frequencies of CD14+ monocytic MDSCs in newly diagnosed AML that co-express CD33 but lack HLA-DR (HLA-DRlo). AML-blasts induce HLA-DRlo cells from healthy donor-derived monocytes in vitro that suppress T-cells and express indoleamine-2,3-dioxygenase (IDO). We investigated whether a CD33/CD3-bispecific BiTE® antibody construct (AMG 330) with pre-clinical activity against AML-blasts by redirection of T-cells can eradicate CD33+ MDSCs. In fact, T-cells eliminate IDO+CD33+ MDSCs in the presence of AMG 330. Depletion of total CD14+ cells (including MDSCs) in peripheral blood mononuclear cells from AML patients did not enhance AMG 330-triggered T-cell activation and expansion, but boosted AML-blast lysis. This finding was corroborated in experiments showing that adding MDSCs into co-cultures of T- and AML-cells reduced AML-blast killing, while IDO inhibition promotes AMG 330-mediated clearance of AML-blasts. Taken together, our results suggest that AMG 330 may achieve anti-leukemic efficacy not only through T-cell-mediated cytotoxicity against AML-blasts but also against CD33+ MDSCs, suggesting that it is worth exploring the predictive role of MDSCs for responsiveness towards an AMG 330-based therapy.
Keywords: Acute myeloid leukemia; Bispecific antibodies; Myeloid derived suppressor cells.
Conflict of interest statement
Ethics approval and consent to participate
All samples were collected upon approval by the local ethics committee (number: 3779/138_17B) and patients’ informed consent.
Consent for publication
Not applicable.
Competing interests
R.K. and M.L. are employed by Amgen Research (Munich) GmbH. C.D.S. is employed by Amgen Inc. R.J., A.M., and D.M. were supported by research funding from Amgen Inc. The remaining authors declare no conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Immunosuppressive role of CD11b+ CD33+ HLA-DR- myeloid-derived suppressor cells-like blast subpopulation in acute myeloid leukemia.Cancer Med. 2020 Oct;9(19):7007-7017. doi: 10.1002/cam4.3360. Epub 2020 Aug 11. Cancer Med. 2020. PMID: 32780544 Free PMC article.
-
CD33 target validation and sustained depletion of AML blasts in long-term cultures by the bispecific T-cell-engaging antibody AMG 330.Blood. 2014 Jan 16;123(3):356-65. doi: 10.1182/blood-2013-08-523548. Epub 2013 Dec 3. Blood. 2014. PMID: 24300852
-
The Broad Anti-AML Activity of the CD33/CD3 BiTE Antibody Construct, AMG 330, Is Impacted by Disease Stage and Risk.PLoS One. 2015 Aug 25;10(8):e0135945. doi: 10.1371/journal.pone.0135945. eCollection 2015. PLoS One. 2015. PMID: 26305211 Free PMC article.
-
Clinical overview of anti-CD19 BiTE(®) and ex vivo data from anti-CD33 BiTE(®) as examples for retargeting T cells in hematologic malignancies.Mol Immunol. 2015 Oct;67(2 Pt A):58-66. doi: 10.1016/j.molimm.2015.02.033. Epub 2015 Apr 13. Mol Immunol. 2015. PMID: 25883042 Review.
-
Harnessing the Immune System Against Leukemia: Monoclonal Antibodies and Checkpoint Strategies for AML.Adv Exp Med Biol. 2017;995:73-95. doi: 10.1007/978-3-319-53156-4_4. Adv Exp Med Biol. 2017. PMID: 28321813 Review.
Cited by
-
Bispecific Antibodies in Hematological Malignancies: A Scoping Review.Cancers (Basel). 2023 Sep 14;15(18):4550. doi: 10.3390/cancers15184550. Cancers (Basel). 2023. PMID: 37760519 Free PMC article. Review.
-
CombiFlow: combinatorial AML-specific plasma membrane expression profiles allow longitudinal tracking of clones.Blood Adv. 2022 Apr 12;6(7):2129-2143. doi: 10.1182/bloodadvances.2021005018. Blood Adv. 2022. PMID: 34543390 Free PMC article.
-
How I treat high-risk acute myeloid leukemia using preemptive adoptive cellular immunotherapy.Blood. 2023 Jan 5;141(1):22-38. doi: 10.1182/blood.2021012411. Blood. 2023. PMID: 35512203 Free PMC article.
-
Targeting the Tumor Microenvironment in Acute Myeloid Leukemia: The Future of Immunotherapy and Natural Products.Biomedicines. 2022 Jun 14;10(6):1410. doi: 10.3390/biomedicines10061410. Biomedicines. 2022. PMID: 35740430 Free PMC article. Review.
-
Resistance to Hypomethylating Agents in Myelodysplastic Syndrome and Acute Myeloid Leukemia From Clinical Data and Molecular Mechanism.Front Oncol. 2021 Sep 28;11:706030. doi: 10.3389/fonc.2021.706030. eCollection 2021. Front Oncol. 2021. PMID: 34650913 Free PMC article. Review.
References
-
- Gaiger A, Reese V, Disis ML, Cheever MA. Immunity to WT1 in the animal model and in patients with acute myeloid leukemia. Blood. 2000;96(4):1480–1489. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
