A variably imprinted epiallele impacts seed development
- PMID: 30395602
- PMCID: PMC6237401
- DOI: 10.1371/journal.pgen.1007469
A variably imprinted epiallele impacts seed development
Abstract
The contribution of epigenetic variation to phenotypic variation is unclear. Imprinted genes, because of their strong association with epigenetic modifications, represent an opportunity for the discovery of such phenomena. In mammals and flowering plants, a subset of genes are expressed from only one parental allele in a process called gene imprinting. Imprinting is associated with differential DNA methylation and chromatin modifications between parental alleles. In flowering plants imprinting occurs in a seed tissue - endosperm. Proper endosperm development is essential for the production of viable seeds. We previously showed that in Arabidopsis thaliana intraspecific imprinting variation is correlated with naturally occurring DNA methylation polymorphisms. Here, we investigated the mechanisms and function of allele-specific imprinting of the class IV homeodomain leucine zipper (HD-ZIP) transcription factor HDG3. In imprinted strains, HDG3 is expressed primarily from the methylated paternally inherited allele. We manipulated the methylation state of endogenous HDG3 in a non-imprinted strain and demonstrated that methylation of a proximal transposable element is sufficient to promote HDG3 expression and imprinting. Gain of HDG3 imprinting was associated with earlier endosperm cellularization and changes in seed weight. These results indicate that epigenetic variation alone is sufficient to explain imprinting variation and demonstrate that epialleles can underlie variation in seed development phenotypes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
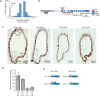
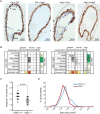

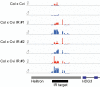
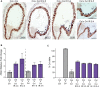
Similar articles
-
Natural epigenetic polymorphisms lead to intraspecific variation in Arabidopsis gene imprinting.Elife. 2014 Jul 3;3:e03198. doi: 10.7554/eLife.03198. Elife. 2014. PMID: 24994762 Free PMC article.
-
Both maternally and paternally imprinted genes regulate seed development in rice.New Phytol. 2017 Oct;216(2):373-387. doi: 10.1111/nph.14510. Epub 2017 Mar 13. New Phytol. 2017. PMID: 28295376
-
Identification of imprinted genes subject to parent-of-origin specific expression in Arabidopsis thaliana seeds.BMC Plant Biol. 2011 Aug 12;11:113. doi: 10.1186/1471-2229-11-113. BMC Plant Biol. 2011. PMID: 21838868 Free PMC article.
-
Evolution, function, and regulation of genomic imprinting in plant seed development.J Exp Bot. 2012 Aug;63(13):4713-22. doi: 10.1093/jxb/ers145. J Exp Bot. 2012. PMID: 22922638 Review.
-
Genomic imprinting during seed development.Adv Genet. 2002;46:165-214. doi: 10.1016/s0065-2660(02)46007-5. Adv Genet. 2002. PMID: 11931224 Review.
Cited by
-
United by conflict: Convergent signatures of parental conflict in angiosperms and placental mammals.J Hered. 2024 Oct 23;115(6):625-642. doi: 10.1093/jhered/esae009. J Hered. 2024. PMID: 38366852 Review.
-
A role for worm cutl-24 in background- and parent-of-origin-dependent ER stress resistance.BMC Genomics. 2022 Dec 20;23(1):842. doi: 10.1186/s12864-022-09063-w. BMC Genomics. 2022. PMID: 36539699 Free PMC article.
-
How Stress Facilitates Phenotypic Innovation Through Epigenetic Diversity.Front Plant Sci. 2021 Jan 15;11:606800. doi: 10.3389/fpls.2020.606800. eCollection 2020. Front Plant Sci. 2021. PMID: 33519857 Free PMC article.
-
Regulation of regeneration in Arabidopsis thaliana.aBIOTECH. 2023 Nov 22;4(4):332-351. doi: 10.1007/s42994-023-00121-9. eCollection 2023 Dec. aBIOTECH. 2023. PMID: 38106435 Free PMC article.
-
Canalization of genome-wide transcriptional activity in Arabidopsis thaliana accessions by MET1-dependent CG methylation.Genome Biol. 2022 Dec 20;23(1):263. doi: 10.1186/s13059-022-02833-5. Genome Biol. 2022. PMID: 36539836 Free PMC article.
References
-
- Gehring M, Huh JH, Hsieh T-F, Penterman J, Choi Y, Harada JJ, et al. DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation. Cell. 2006; 124(3):495–506. 10.1016/j.cell.2005.12.034 - DOI - PMC - PubMed
-
- Gehring M, Bubb KL, Henikoff S. Extensive demethylation of repetitive elements during seed development underlies gene imprinting. Science. 2009; 324(5933):1447–51. 10.1126/science.1171609 - DOI - PMC - PubMed
-
- Hsieh T-F, Ibarra CA, Silva P, Zemach A, Eshed-Williams L, Fischer RL, et al. Genome-wide demethylation of Arabidopsis endosperm. Science. 2009; 324(5933):1451–4. 10.1126/science.1172417 - DOI - PMC - PubMed
-
- Satyaki PRV, Gehring M. DNA methylation and imprinting in plants: machinery and mechanisms. Crit Rev Biochem Mol Biol. 2017; 52(2):163–75. 10.1080/10409238.2017.1279119 - DOI - PubMed
-
- Haig D. The kinship theory of genomic imprinting. Annu Rev Ecol Syst. 2000; 31: 9–32.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

