The role of tumour microenvironment: a new vision for cholangiocarcinoma
- PMID: 30394682
- PMCID: PMC6307844
- DOI: 10.1111/jcmm.13953
The role of tumour microenvironment: a new vision for cholangiocarcinoma
Abstract
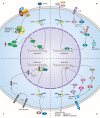
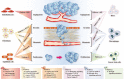
Cholangiocarcinoma (CCA) is a relatively rare malignant and lethal tumour derived from bile duct epithelium and the morbidity is now increasing worldwide. This disease is difficult to diagnose at its inchoate stage and has poor prognosis. Therefore, a clear understanding of pathogenesis and major influencing factors is the key to develop effective therapeutic methods for CCA. In previous studies, canonical correlation analysis has demonstrated that tumour microenvironment plays an intricate role in the progression of various types of cancers including CCA. CCA tumour microenvironment is a dynamic environment consisting of authoritative tumour stromal cells and extracellular matrix where tumour stromal cells and cancer cells can thrive. CCA stromal cells include immune and non-immune cells, such as inflammatory cells, endothelial cells, fibroblasts, and macrophages. Likewise, CCA tumour microenvironment contains abundant proliferative factors and can significantly impact the behaviour of cancer cells. Through abominably intricate interactions with CCA cells, CCA tumour microenvironment plays an important role in promoting tumour proliferation, accelerating neovascularization, facilitating tumour invasion, and preventing tumour cells from organismal immune reactions and apoptosis. This review summarizes the recent research progress regarding the connection between tumour behaviours and tumour stromal cells in CCA, as well as the mechanism underlying the effect of tumour stromal cells on the growth of CCA. A thorough understanding of the relationship between CCA and tumour stromal cells can shed some light on the development of new therapeutic methods for treating CCA.
Keywords: cholangiocarcinoma; tumor microenvironment; tumor stromal cells.
© 2018 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures
Similar articles
-
The immune milieu of cholangiocarcinoma: From molecular pathogenesis to precision medicine.J Autoimmun. 2019 Jun;100:17-26. doi: 10.1016/j.jaut.2019.03.007. Epub 2019 Mar 9. J Autoimmun. 2019. PMID: 30862450 Review.
-
The tumour microenvironment and immune milieu of cholangiocarcinoma.Liver Int. 2019 May;39 Suppl 1(Suppl 1):63-78. doi: 10.1111/liv.14098. Liver Int. 2019. PMID: 30907492 Free PMC article. Review.
-
Impact of microenvironment and stem-like plasticity in cholangiocarcinoma: molecular networks and biological concepts.J Hepatol. 2015 Jan;62(1):198-207. doi: 10.1016/j.jhep.2014.09.007. Epub 2014 Sep 16. J Hepatol. 2015. PMID: 25220250 Review.
-
The Tumor Immune Microenvironment plays a Key Role in Driving the Progression of Cholangiocarcinoma.Curr Cancer Drug Targets. 2024;24(7):681-700. doi: 10.2174/0115680096267791231115101107. Curr Cancer Drug Targets. 2024. PMID: 38213139 Review.
-
Experimental models to unravel the molecular pathogenesis, cell of origin and stem cell properties of cholangiocarcinoma.Liver Int. 2019 May;39 Suppl 1:79-97. doi: 10.1111/liv.14094. Liver Int. 2019. PMID: 30851232 Review.
Cited by
-
Injured Endothelial Cell: A Risk Factor for Pulmonary Fibrosis.Int J Mol Sci. 2023 May 14;24(10):8749. doi: 10.3390/ijms24108749. Int J Mol Sci. 2023. PMID: 37240093 Free PMC article. Review.
-
A randomized placebo-controlled phase I clinical trial to evaluate the immunomodulatory activities of Atractylodes lancea (Thunb) DC. in healthy Thai subjects.BMC Complement Med Ther. 2021 Feb 12;21(1):61. doi: 10.1186/s12906-020-03199-6. BMC Complement Med Ther. 2021. PMID: 33579265 Free PMC article. Clinical Trial.
-
Cholangiocarcinoma: novel therapeutic targets.Expert Opin Ther Targets. 2020 Apr;24(4):345-357. doi: 10.1080/14728222.2020.1733528. Epub 2020 Feb 26. Expert Opin Ther Targets. 2020. PMID: 32077341 Free PMC article. Review.
-
Role of Chemokines in the Biology of Cholangiocarcinoma.Cancers (Basel). 2020 Aug 7;12(8):2215. doi: 10.3390/cancers12082215. Cancers (Basel). 2020. PMID: 32784743 Free PMC article. Review.
-
Biliary Strictures and Cholangiocarcinoma - Untangling a Diagnostic Conundrum.Front Oncol. 2021 Sep 30;11:699401. doi: 10.3389/fonc.2021.699401. eCollection 2021. Front Oncol. 2021. PMID: 34660269 Free PMC article. Review.
References
-
- Claessen MM, Vleggaar FP, Tytgat KM, et al. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol. 2009;50:158‐164. - PubMed
-
- Shaib YH, El‐Serag HB, Davila JA, et al. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case‐control study. Gastroenterology. 2005;128:620‐626. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

