A Cinderella story: how the vacuolar proteases Pep4 and Prb1 do more than cleaning up the cell's mass degradation processes
- PMID: 30386788
- PMCID: PMC6206407
- DOI: 10.15698/mic2018.10.650
A Cinderella story: how the vacuolar proteases Pep4 and Prb1 do more than cleaning up the cell's mass degradation processes
Abstract
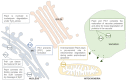
Recently, several research groups have assigned non-vacuolar functions to the well-known Saccharomyces cerevisiae vacuolar proteases Pep4 and Prb1, which are also known as proteinases A and B. These non-vacuolar activities seem to be autophagy-independent and stress-induced and suggest an unexplored but possibly prominent role for the proteases outside the vacuole. The functions range from the involvement in programmed cell death, to protection from hazardous protein forms and regulation of gene expression. We propose that a deeper understanding of these molecular processes will provide new insights that will be important for both fungal biology as well as studies in mammalian cells, as they might open up perspectives in the search for novel drug targets. To illustrate this, we summarize the recent literature on non-vacuolar Pep4 and Prb1 functions in S. cerevisiae and review the current data on the protein homologs in pathogenic fungi.
Keywords: Pep4; Prb1; SAGA; Saccharomyces cerevisiae; cathepsin D; prion; programmed cell death; protease.
Conflict of interest statement
Conflict of interest: The authors declare no conflict of interest.
Figures
Similar articles
-
Vacuolar proteases and autophagy in phytopathogenic fungi: A review.Front Fungal Biol. 2022 Oct 26;3:948477. doi: 10.3389/ffunb.2022.948477. eCollection 2022. Front Fungal Biol. 2022. PMID: 37746183 Free PMC article. Review.
-
Vacuolar and extracellular maturation of Saccharomyces cerevisiae proteinase A.Yeast. 1996 Jul;12(9):823-32. doi: 10.1002/(SICI)1097-0061(199607)12:9%3C823::AID-YEA975%3E3.0.CO;2-J. Yeast. 1996. PMID: 8840499
-
The mechanism of Atg15-mediated membrane disruption in autophagy.J Cell Biol. 2023 Dec 4;222(12):e202306120. doi: 10.1083/jcb.202306120. Epub 2023 Nov 2. J Cell Biol. 2023. PMID: 37917025 Free PMC article.
-
Vacuolar hydrolysis and efflux: current knowledge and unanswered questions.Autophagy. 2019 Feb;15(2):212-227. doi: 10.1080/15548627.2018.1545821. Epub 2018 Nov 22. Autophagy. 2019. PMID: 30422029 Free PMC article. Review.
-
Biogenesis of the yeast vacuole (lysosome). The use of active-site mutants of proteinase yscA to determine the necessity of the enzyme for vacuolar proteinase maturation and proteinase yscB stability.Eur J Biochem. 1995 Jul 1;231(1):115-25. doi: 10.1111/j.1432-1033.1995.tb20677.x. Eur J Biochem. 1995. PMID: 7628461
Cited by
-
A multistrategy approach for improving the expression of E. coli phytase in Pichia pastoris.J Ind Microbiol Biotechnol. 2020 Dec;47(12):1161-1172. doi: 10.1007/s10295-020-02311-6. Epub 2020 Sep 15. J Ind Microbiol Biotechnol. 2020. PMID: 32935229
-
Characterization of Aspartic Proteases from Paracoccidioides brasiliensis and Their Role in Fungal Thermo-Dimorphism.J Fungi (Basel). 2023 Mar 19;9(3):375. doi: 10.3390/jof9030375. J Fungi (Basel). 2023. PMID: 36983543 Free PMC article.
-
Cellular models of Batten disease.Biochim Biophys Acta Mol Basis Dis. 2020 Sep 1;1866(9):165559. doi: 10.1016/j.bbadis.2019.165559. Epub 2019 Oct 23. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 31655107 Free PMC article. Review.
-
Vacuolar proteases and autophagy in phytopathogenic fungi: A review.Front Fungal Biol. 2022 Oct 26;3:948477. doi: 10.3389/ffunb.2022.948477. eCollection 2022. Front Fungal Biol. 2022. PMID: 37746183 Free PMC article. Review.
-
The expression system influences stability, maturation efficiency, and oligomeric properties of the potassium-chloride co-transporter KCC2.Neurochem Int. 2024 Mar;174:105695. doi: 10.1016/j.neuint.2024.105695. Epub 2024 Feb 17. Neurochem Int. 2024. PMID: 38373478
References
-
- Galluzzi L, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cecconi F, Choi AM, Chu CT, Codogno P, Colombo MI, Cuervo AM, Debnath J, Deretic V, Dikic I, Eskelinen EL, Fimia GM, Fulda S, Gewirtz DA, Green DR, Hansen M, Harper JW, Jaattela M, Johansen T, Juhasz G, Kimmelman AC, Kraft C, Ktistakis NT, Kumar S, Levine B, Lopez-Otin C. Molecular definitions of autophagy and related processes. EMBO J. 2017;36(13):1811–1836. doi: 10.15252/embj.201796697. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
