SIRT1 Is a Potential Drug Target for Treatment of Diabetic Kidney Disease
- PMID: 30386303
- PMCID: PMC6199382
- DOI: 10.3389/fendo.2018.00624
SIRT1 Is a Potential Drug Target for Treatment of Diabetic Kidney Disease
Abstract
Multiple studies have demonstrated a critical role of Sirtuin-1 (SIRT1) deacetylase in protecting kidney cells from cellular stresses. A protective role of SIRT1 has been reported in both podocytes and renal tubular cells in multiple kidney disease settings, including diabetic kidney disease (DKD). We and others have shown that SIRT1 exerts renoprotective effects in DKD in part through the deacetylation of transcription factors involved in the disease pathogenesis, such as p53, FOXO, RelA/p65NF-κB, STAT3, and PGC1α/PPARγ. Recently we showed that the podocyte-specific overexpression of SIRT1 attenuated proteinuria and kidney injury in an experimental model of DKD, further confirming SIRT1 as a potential target to treat kidney disease. Known agonists of SIRT1 such as resveratrol diminished diabetic kidney injury in several animal models. Similarly, we also showed that puerarin, a Chinese herbal medicine compound, activates SIRT1 to provide renoprotection in mouse models of DKD. However, as these are non-specific SIRT1 agonists, we recently developed a more specific and potent SIRT1 agonist (BF175) that significantly attenuated diabetic kidney injury in type 1 diabetic OVE26 mice. We also previously reported that MS417, a bromodomain inhibitor that disrupts the interaction between the acetyl-residues of NF-κB and bromodomain-containing protein 4 (BRD4) also attenuates DKD. These results suggest that SIRT1 agonists and bromodomain inhibitors could be potential new therapuetic treatments against DKD progression.
Keywords: SIRT1; acetylation; bromodomain inhibitor; diabetic kidney disease; podocytes.
Figures
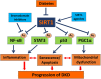
 ): stimulation; (
): stimulation; ( ): inhibition; (
): inhibition; ( ): interaction.
): interaction.Similar articles
-
Increased podocyte Sirtuin-1 function attenuates diabetic kidney injury.Kidney Int. 2018 Jun;93(6):1330-1343. doi: 10.1016/j.kint.2017.12.008. Epub 2018 Feb 22. Kidney Int. 2018. PMID: 29477240 Free PMC article.
-
Role of transcription factor acetylation in diabetic kidney disease.Diabetes. 2014 Jul;63(7):2440-53. doi: 10.2337/db13-1810. Epub 2014 Mar 7. Diabetes. 2014. PMID: 24608443 Free PMC article.
-
SIRT1: Mechanism and Protective Effect in Diabetic Nephropathy.Endocr Metab Immune Disord Drug Targets. 2021;21(5):835-842. doi: 10.2174/1871530320666201029143606. Endocr Metab Immune Disord Drug Targets. 2021. PMID: 33121427 Review.
-
Reduction in podocyte SIRT1 accelerates kidney injury in aging mice.Am J Physiol Renal Physiol. 2017 Sep 1;313(3):F621-F628. doi: 10.1152/ajprenal.00255.2017. Epub 2017 Jun 14. Am J Physiol Renal Physiol. 2017. PMID: 28615249 Free PMC article.
-
Epigenetic Regulation Through SIRT1 in Podocytes.Curr Hypertens Rev. 2016;12(2):89-94. doi: 10.2174/1573402112666160302102515. Curr Hypertens Rev. 2016. PMID: 26931472 Review.
Cited by
-
Molecular Challenges and Opportunities in Climate Change-Induced Kidney Diseases.Biomolecules. 2024 Feb 21;14(3):251. doi: 10.3390/biom14030251. Biomolecules. 2024. PMID: 38540672 Free PMC article. Review.
-
The complicated role of mitochondria in the podocyte.Am J Physiol Renal Physiol. 2020 Dec 1;319(6):F955-F965. doi: 10.1152/ajprenal.00393.2020. Epub 2020 Oct 19. Am J Physiol Renal Physiol. 2020. PMID: 33073585 Free PMC article.
-
The role of N6-methyladenosine (m6A) in kidney diseases.Front Med (Lausanne). 2023 Sep 28;10:1247690. doi: 10.3389/fmed.2023.1247690. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37841018 Free PMC article. Review.
-
Molineria recurvata Ameliorates Streptozotocin-Induced Diabetic Nephropathy through Antioxidant and Anti-Inflammatory Pathways.Molecules. 2022 Aug 5;27(15):4985. doi: 10.3390/molecules27154985. Molecules. 2022. PMID: 35956936 Free PMC article.
-
Regulation of autophagy by natural polyphenols in the treatment of diabetic kidney disease: therapeutic potential and mechanism.Front Endocrinol (Lausanne). 2023 Aug 10;14:1142276. doi: 10.3389/fendo.2023.1142276. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37635982 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

