Dietary ω-3 fatty acids alter the lipid mediator profile and alleviate allergic conjunctivitis without modulating Th2 immune responses
- PMID: 30383446
- PMCID: PMC6404575
- DOI: 10.1096/fj.201801805R
Dietary ω-3 fatty acids alter the lipid mediator profile and alleviate allergic conjunctivitis without modulating Th2 immune responses
Abstract
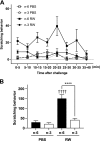
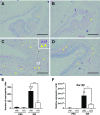
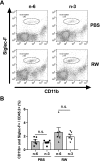
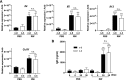
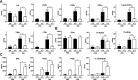
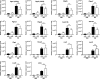
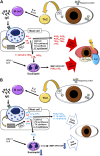
Allergic conjunctivitis (AC) is one of the most common ocular surface diseases in the world. In AC, T helper type 2 (Th2) immune responses play central roles in orchestrating inflammatory responses. However, the roles of lipid mediators in the onset and progression of AC remain to be fully explored. Although previous reports have shown the beneficial effects of supplementation of ω-3 fatty acids in asthma or atopic dermatitis, the underlying molecular mechanisms are poorly understood. In this study, a diet rich in ω-3 fatty acids alleviated AC symptoms in both early and late phases without affecting Th2 immune responses, but rather by altering the lipid mediator profiles. The ω-3 fatty acids completely suppressed scratching behavior toward the eyes, an allergic reaction provoked by itch. Although total serum IgE levels and the expression levels of Th2 cytokines and chemokines in the conjunctiva were not altered by ω-3 fatty acids, eosinophil infiltration into the conjunctiva was dramatically suppressed. The levels of ω-6-derived proinflammatory lipid mediators, including those with chemoattractant properties for eosinophils, were markedly reduced in the conjunctivae of ω-3 diet-fed mice. Dietary ω-3 fatty acids can alleviate a variety of symptoms of AC by altering the lipid mediator profile.-Hirakata, T., Lee, H.-C., Ohba, M., Saeki, K., Okuno, T., Murakami, A., Matsuda, A., Yokomizo, T. Dietary ω-3 fatty acids alter the lipid mediator profile and alleviate allergic conjunctivitis without modulating Th2 immune responses.
Keywords: eicosanoid; fish oil; hay fever; inflammation; lipidomics.
Conflict of interest statement
The authors thank members of the Laboratory of Morphology and Image Analysis Research Support Center at the Juntendo University Graduate School of Medicine for technical assistance with microscopy. This work was supported by the Ministry of Education, Culture, Sports, Science, and Technology (MEXT)/Japan Society for the Promotion of Sport Grants-in-Aid for Scientific Research (KAKENHI) (18K16246 to H.-C.L.; 15K08316 and 18K06923 to K.S.; 15KK0320 and 16K08596 to T.O.; 16K11303 to A.Matsuda; and 15H05904, 15H04708, and 18H02627 to T.Y.), and grants from the Naito Foundation, the Ono Medical Research Foundation, the Uehara Memorial Foundation, the Mitsubishi Foundation, the Takeda Science Foundation, and, in part, by Grant-in-Aid S1311011 from the Foundation of Strategic Research Projects in Private Universities from MEXT, and institutional grants for Environmental and Gender-Specific Medicine and the Atopy Research Center at the Juntendo University Graduate School of Medicine. The authors declare no conflicts of interest.
Figures

Similar articles
-
Long-chain polyunsaturated fatty acids are consumed during allergic inflammation and affect T helper type 1 (Th1)- and Th2-mediated hypersensitivity differently.Clin Exp Immunol. 2010 Jun;160(3):411-9. doi: 10.1111/j.1365-2249.2010.04107.x. Epub 2010 Feb 10. Clin Exp Immunol. 2010. PMID: 20148912 Free PMC article.
-
Superoxide dismutase 3 attenuates experimental Th2-driven allergic conjunctivitis.Clin Immunol. 2017 Mar;176:49-54. doi: 10.1016/j.clim.2016.12.010. Epub 2017 Jan 4. Clin Immunol. 2017. PMID: 28063939
-
The roles of omega-3 fatty acids and resolvins in allergic conjunctivitis.Curr Opin Allergy Clin Immunol. 2019 Oct;19(5):517-525. doi: 10.1097/ACI.0000000000000561. Curr Opin Allergy Clin Immunol. 2019. PMID: 31465315 Review.
-
Rapamycin attenuates Th2-driven experimental allergic conjunctivitis.Clin Immunol. 2018 May;190:1-10. doi: 10.1016/j.clim.2018.02.004. Epub 2018 Feb 9. Clin Immunol. 2018. PMID: 29432811
-
Dietary fatty acids and allergy.Ann Med. 1999 Aug;31(4):282-7. doi: 10.3109/07853899908995891. Ann Med. 1999. PMID: 10480759 Review.
Cited by
-
Sex-based differences in conjunctival goblet cell responses to pro-inflammatory and pro-resolving mediators.Sci Rep. 2022 Sep 29;12(1):16305. doi: 10.1038/s41598-022-20177-9. Sci Rep. 2022. PMID: 36175572 Free PMC article.
-
Production profile of lipid mediators in conjunctival lavage fluid in allergic and infectious conjunctivitis in guinea pigs.Front Allergy. 2023 Jul 6;4:1218447. doi: 10.3389/falgy.2023.1218447. eCollection 2023. Front Allergy. 2023. PMID: 37483465 Free PMC article.
-
Host- and Microbe-Dependent Dietary Lipid Metabolism in the Control of Allergy, Inflammation, and Immunity.Front Nutr. 2019 Apr 10;6:36. doi: 10.3389/fnut.2019.00036. eCollection 2019. Front Nutr. 2019. PMID: 31024921 Free PMC article. Review.
-
Vernal Keratoconjunctivitis: Immunopathological Insights and Therapeutic Applications of Immunomodulators.Life (Basel). 2024 Mar 9;14(3):361. doi: 10.3390/life14030361. Life (Basel). 2024. PMID: 38541686 Free PMC article. Review.
-
Resolvin E1 Reduces Leukotriene B4-Induced Intracellular Calcium Increase and Mucin Secretion in Rat Conjunctival Goblet Cells.Am J Pathol. 2020 Sep;190(9):1823-1832. doi: 10.1016/j.ajpath.2020.06.001. Epub 2020 Jun 16. Am J Pathol. 2020. PMID: 32561135 Free PMC article.
References
-
- Singh K., Axelrod S., Bielory L. (2010) The epidemiology of ocular and nasal allergy in the United States, 1988-1994. J. Allergy. Clin. Immunol. 126, 778–783.e6 - PubMed
-
- Ono S. J., Abelson M. B. (2005) Allergic conjunctivitis: update on pathophysiology and prospects for future treatment. J. Allergy Clin. Immunol. 115, 118–122 - PubMed
-
- Keane-Myers A. (2001) The pathogenesis of allergic conjunctivitis. Curr. Allergy Asthma Rep. 1, 550–557 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

