Inhibition of Epstein-Barr Virus Replication in Human Papillomavirus-Immortalized Keratinocytes
- PMID: 30381489
- PMCID: PMC6321917
- DOI: 10.1128/JVI.01216-18
Inhibition of Epstein-Barr Virus Replication in Human Papillomavirus-Immortalized Keratinocytes
Abstract
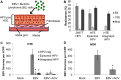
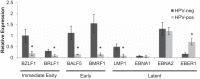
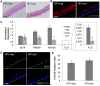
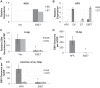
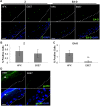
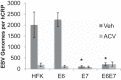
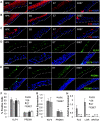
Epstein-Barr virus (EBV) is implicated in the pathogenesis of human papillomavirus (HPV)-associated oropharyngeal squamous cell carcinoma (OSCC). EBV-associated cancers harbor a latent EBV infection characterized by a lack of viral replication and the expression of viral oncogenes. Cellular changes promoted by HPV are comparable to those shown to facilitate EBV latency, though whether HPV-positive cells support a latent EBV infection has not been demonstrated. Using a model of direct EBV infection into HPV16-immortalized tonsillar cells grown in organotypic raft culture, we showed robust EBV replication in HPV-negative rafts but little to no replication in HPV-immortalized rafts. The reduced EBV replication was independent of immortalization, as human telomerase-immortalized normal oral keratinocytes supported robust EBV replication. Furthermore, we observed reduced EBV lytic gene expression and increased expression of EBER1, a noncoding RNA highly expressed in latently infected cells, in the presence of HPV. The use of human foreskin keratinocyte rafts expressing the HPV16 E6 and/or E7 oncogene(s) (HPV E6 and E7 rafts) showed that E7 was sufficient to reduce EBV replication. EBV replication is dependent upon epithelial differentiation and the differentiation-dependent expression of the transcription factors KLF4 and PRDM1. While KLF4 and PRDM1 levels were unaltered, the expression levels of KLF4 transcriptional targets, including late differentiation markers, were reduced in HPV E6 and E7 rafts compared to their levels in parental rafts. However, the HPV E7-mediated block in EBV replication correlated with delayed expression of early differentiation markers. Overall, this study reveals an HPV16-mediated block in EBV replication, through E7, that may facilitate EBV latency and long-term persistence in the tumor context.IMPORTANCE Using a model examining the establishment of EBV infection in HPV-immortalized tissues, we showed an HPV-induced interruption of the normal EBV life cycle reminiscent of a latent EBV infection. Our data support the notion that a persistent EBV epithelial infection depends upon preexisting cellular alterations and suggest the ability of HPV to promote such changes. More importantly, these findings introduce a model for how EBV coinfection may influence HPV-positive (HPV-pos) OSCC pathogenesis. Latently EBV-infected epithelial cells, as well as other EBV-associated head-and-neck carcinomas, exhibit oncogenic phenotypes commonly seen in HPV-pos OSCC. Therefore, an HPV-induced shift in the EBV life cycle toward latency would not only facilitate EBV persistence but also provide additional viral oncogene expression, which can contribute to the rapid progression of HPV-pos OSCC. These findings provide a step toward defining a role for EBV as a cofactor in HPV-positive oropharyngeal tumors.
Keywords: EBV; Epstein-Barr virus; HPV; latency; organotypic; replication; viral replication.
Copyright © 2019 American Society for Microbiology.
Figures

Similar articles
-
Retinoblastoma Protein Is Required for Epstein-Barr Virus Replication in Differentiated Epithelia.J Virol. 2023 Feb 28;97(2):e0103222. doi: 10.1128/jvi.01032-22. Epub 2023 Jan 31. J Virol. 2023. PMID: 36719239 Free PMC article.
-
Epstein-Barr virus replication within differentiated epithelia requires pRb sequestration of activator E2F transcription factors.J Virol. 2024 Oct 22;98(10):e0099524. doi: 10.1128/jvi.00995-24. Epub 2024 Sep 18. J Virol. 2024. PMID: 39291960
-
Genome-Wide Transcriptome Analysis of Human Papillomavirus 16-Infected Primary Keratinocytes Reveals Subtle Perturbations Mostly due to E7 Protein Expression.J Virol. 2020 Jan 17;94(3):e01360-19. doi: 10.1128/JVI.01360-19. Print 2020 Jan 17. J Virol. 2020. PMID: 31748387 Free PMC article.
-
Manipulation of Epithelial Differentiation by HPV Oncoproteins.Viruses. 2019 Apr 22;11(4):369. doi: 10.3390/v11040369. Viruses. 2019. PMID: 31013597 Free PMC article. Review.
-
Pre-malignant nasopharyngeal epithelial cell models.Ai Zheng. 2009 Oct;28(10):1012-5. doi: 10.5732/cjc.009.10496. Ai Zheng. 2009. PMID: 19799806 Review.
Cited by
-
An Exonuclease V-qPCR Assay to Analyze the State of the Human Papillomavirus 16 Genome in Cell Lines and Tissues.Curr Protoc Microbiol. 2020 Dec;59(1):e119. doi: 10.1002/cpmc.119. Curr Protoc Microbiol. 2020. PMID: 33064937 Free PMC article.
-
Detecting episomal or integrated human papillomavirus 16 DNA using an exonuclease V-qPCR-based assay.Virology. 2019 Nov;537:149-156. doi: 10.1016/j.virol.2019.08.021. Epub 2019 Aug 22. Virology. 2019. PMID: 31493653 Free PMC article.
-
High-Risk Human Papillomavirus and Epstein-Barr Virus Coinfection: A Potential Role in Head and Neck Carcinogenesis.Biology (Basel). 2021 Nov 26;10(12):1232. doi: 10.3390/biology10121232. Biology (Basel). 2021. PMID: 34943147 Free PMC article. Review.
-
Retinoblastoma Protein Is Required for Epstein-Barr Virus Replication in Differentiated Epithelia.J Virol. 2023 Feb 28;97(2):e0103222. doi: 10.1128/jvi.01032-22. Epub 2023 Jan 31. J Virol. 2023. PMID: 36719239 Free PMC article.
-
The environmental pollutant and tobacco smoke constituent dibenzo[def,p]chrysene is a co-factor for malignant progression of mouse oral papillomavirus infections.Chem Biol Interact. 2021 Jan 5;333:109321. doi: 10.1016/j.cbi.2020.109321. Epub 2020 Nov 10. Chem Biol Interact. 2021. PMID: 33186600 Free PMC article.
References
-
- Dahlstrom KR, Adler-Storthz K, Etzel CJ, Liu Z, Dillon L, El-Naggar AK, Spitz MR, Schiller JT, Wei Q, Sturgis EM. 2003. Human papillomavirus type 16 infection and squamous cell carcinoma of the head and neck in never-smokers: a matched pair analysis. Clin Cancer Res 9:2620–2626. - PubMed
-
- Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D. 2000. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 92:709–720. doi:10.1093/jnci/92.9.709. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

