Pharmacological Validation of N-Myristoyltransferase as a Drug Target in Leishmania donovani
- PMID: 30380837
- PMCID: PMC6332449
- DOI: 10.1021/acsinfecdis.8b00226
Pharmacological Validation of N-Myristoyltransferase as a Drug Target in Leishmania donovani
Abstract
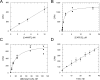
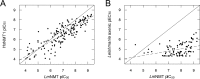
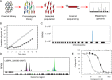
Visceral leishmaniasis (VL), caused by the protozoan parasites Leishmania donovani and L. infantum, is responsible for ∼30 000 deaths annually. Available treatments are inadequate, and there is a pressing need for new therapeutics. N-Myristoyltransferase (NMT) remains one of the few genetically validated drug targets in these parasites. Here, we sought to pharmacologically validate this enzyme in Leishmania. A focused set of 1600 pyrazolyl sulfonamide compounds was screened against L. major NMT in a robust high-throughput biochemical assay. Several potent inhibitors were identified with marginal selectivity over the human enzyme. There was little correlation between the enzyme potency of these inhibitors and their cellular activity against L. donovani axenic amastigotes, and this discrepancy could be due to poor cellular uptake due to the basicity of these compounds. Thus, a series of analogues were synthesized with less basic centers. Although most of these compounds continued to suffer from relatively poor antileishmanial activity, our most potent inhibitor of LmNMT (DDD100097, K i of 0.34 nM) showed modest activity against L. donovani intracellular amastigotes (EC50 of 2.4 μM) and maintained a modest therapeutic window over the human enzyme. Two unbiased approaches, namely, screening against our cosmid-based overexpression library and thermal proteome profiling (TPP), confirm that DDD100097 (compound 2) acts on-target within parasites. Oral dosing with compound 2 resulted in a 52% reduction in parasite burden in our mouse model of VL. Thus, NMT is now a pharmacologically validated target in Leishmania. The challenge in finding drug candidates remains to identify alternative strategies to address the drop-off in activity between enzyme inhibition and in vitro activity while maintaining sufficient selectivity over the human enzyme, both issues that continue to plague studies in this area.
Keywords: Leishmania; N-myristoyltransferase; drug discovery; target validation; thermal proteome profiling (TPP).
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Using a non-image-based medium-throughput assay for screening compounds targeting N-myristoylation in intracellular Leishmania amastigotes.PLoS Negl Trop Dis. 2014 Dec 18;8(12):e3363. doi: 10.1371/journal.pntd.0003363. eCollection 2014 Dec. PLoS Negl Trop Dis. 2014. PMID: 25522361 Free PMC article.
-
A parasite rescue and transformation assay for antileishmanial screening against intracellular Leishmania donovani amastigotes in THP1 human acute monocytic leukemia cell line.J Vis Exp. 2012 Dec 30;(70):4054. doi: 10.3791/4054. J Vis Exp. 2012. PMID: 23299097 Free PMC article.
-
Novel Thienopyrimidine Inhibitors of Leishmania N-Myristoyltransferase with On-Target Activity in Intracellular Amastigotes.J Med Chem. 2020 Jul 23;63(14):7740-7765. doi: 10.1021/acs.jmedchem.0c00570. Epub 2020 Jul 14. J Med Chem. 2020. PMID: 32575985 Free PMC article.
-
Antiparasitic chemotherapy: tinkering with the purine salvage pathway.Adv Exp Med Biol. 2008;625:116-32. doi: 10.1007/978-0-387-77570-8_10. Adv Exp Med Biol. 2008. PMID: 18365663 Review.
-
The preclinical discovery and development of oral miltefosine for the treatment of visceral leishmaniasis: a case history.Expert Opin Drug Discov. 2020 Jun;15(6):647-658. doi: 10.1080/17460441.2020.1743674. Epub 2020 Mar 23. Expert Opin Drug Discov. 2020. PMID: 32202449 Review.
Cited by
-
Omics Approaches in Drug Development against Leishmaniasis: Current Scenario and Future Prospects.Pathogens. 2022 Dec 26;12(1):39. doi: 10.3390/pathogens12010039. Pathogens. 2022. PMID: 36678387 Free PMC article. Review.
-
Identification of Resistance Determinants for a Promising Antileishmanial Oxaborole Series.Microorganisms. 2021 Jun 29;9(7):1408. doi: 10.3390/microorganisms9071408. Microorganisms. 2021. PMID: 34210040 Free PMC article.
-
The antimalarial resistome - finding new drug targets and their modes of action.Curr Opin Microbiol. 2020 Oct;57:49-55. doi: 10.1016/j.mib.2020.06.004. Epub 2020 Jul 15. Curr Opin Microbiol. 2020. PMID: 32682267 Free PMC article. Review.
-
Identification of Potential Leishmania N-Myristoyltransferase Inhibitors from Withania somnifera (L.) Dunal: A Molecular Docking and Molecular Dynamics Investigation.Metabolites. 2023 Jan 6;13(1):93. doi: 10.3390/metabo13010093. Metabolites. 2023. PMID: 36677018 Free PMC article.
-
Screening of medicinal plants unraveled the leishmanicidal credibility of Garcinia cowa; highlighting Norcowanin, a novel anti-leishmanial phytochemical through in-silico study.J Parasit Dis. 2022 Mar;46(1):202-214. doi: 10.1007/s12639-021-01441-7. Epub 2021 Aug 16. J Parasit Dis. 2022. PMID: 35299910 Free PMC article.
References
-
- Nagle A. S.; Khare S.; Kumar A. B.; Supek F.; Buchynskyy A.; Mathison C. J.; Chennamaneni N. K.; Pendem N.; Buckner F. S.; Gelb M. H.; Molteni V. (2014) Recent developments in drug discovery for leishmaniasis and human African trypanosomiasis. Chem. Rev. 114 (22), 11305–11347. 10.1021/cr500365f. - DOI - PMC - PubMed
-
- Ponte-Sucre A.; Gamarro F.; Dujardin J. C.; Barrett M. P.; Lopez-Velez R.; Garcia-Hernandez R.; Pountain A. W.; Mwenechanya R.; Papadopoulou B. (2017) Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Neglected Trop. Dis. 11 (12), e0006052.10.1371/journal.pntd.0006052. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

