Integrated B Cell, Toll-like, and BAFF Receptor Signals Promote Autoantibody Production by Transitional B Cells
- PMID: 30373855
- PMCID: PMC6779322
- DOI: 10.4049/jimmunol.1800393
Integrated B Cell, Toll-like, and BAFF Receptor Signals Promote Autoantibody Production by Transitional B Cells
Abstract
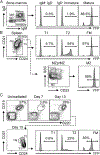
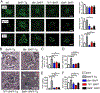
The B cell survival cytokine BAFF has been linked with the pathogenesis of systemic lupus erythematosus (SLE). BAFF binds distinct BAFF-family surface receptors, including the BAFF-R and transmembrane activator and CAML interactor (TACI). Although originally characterized as a negative regulator of B cell activation, TACI signals are critical for class-switched autoantibody (autoAb) production in BAFF transgenic mice. Consistent with this finding, a subset of transitional splenic B cells upregulate surface TACI expression and contribute to BAFF-driven autoAb. In the current study, we interrogated the B cell signals required for transitional B cell TACI expression and Ab production. Surprisingly, despite established roles for dual BCR and TLR signals in autoAb production in SLE, signals downstream of these receptors exerted distinct impacts on transitional B cell TACI expression and autoAb titers. Whereas loss of BCR signals prevented transitional B cell TACI expression and resulted in loss of serum autoAb across all Ig isotypes, lack of TLR signals exerted a more limited impact restricted to autoAb class-switch recombination without altering transitional B cell TACI expression. Finally, in parallel with the protective effect of TACI deletion, loss of BAFF-R signaling also protected against BAFF-driven autoimmunity. Together, these findings highlight how multiple signaling pathways integrate to promote class-switched autoAb production by transitional B cells, events that likely impact the pathogenesis of SLE and other BAFF-dependent autoimmune diseases.
Copyright © 2018 by The American Association of Immunologists, Inc.
Conflict of interest statement
Disclosures
The authors have no financial conflicts of interest.
Figures




Similar articles
-
Deleting the BAFF receptor TACI protects against systemic lupus erythematosus without extensive reduction of B cell numbers.J Autoimmun. 2015 Jul;61:9-16. doi: 10.1016/j.jaut.2015.04.007. Epub 2015 May 29. J Autoimmun. 2015. PMID: 26027434
-
TACI deletion protects against progressive murine lupus nephritis induced by BAFF overexpression.Kidney Int. 2018 Oct;94(4):728-740. doi: 10.1016/j.kint.2018.03.012. Epub 2018 Jun 12. Kidney Int. 2018. PMID: 29907458 Free PMC article.
-
Cutting Edge: BAFF Promotes Autoantibody Production via TACI-Dependent Activation of Transitional B Cells.J Immunol. 2016 May 1;196(9):3525-31. doi: 10.4049/jimmunol.1600017. Epub 2016 Mar 28. J Immunol. 2016. PMID: 27022196 Free PMC article.
-
BAFF receptor and TACI in B-1b cell maintenance and antibacterial responses.Ann N Y Acad Sci. 2015 Dec;1362:57-67. doi: 10.1111/nyas.12772. Epub 2015 May 11. Ann N Y Acad Sci. 2015. PMID: 25962322 Review.
-
BAFF, APRIL and their receptors: structure, function and signaling.Semin Immunol. 2006 Oct;18(5):263-75. doi: 10.1016/j.smim.2006.04.006. Epub 2006 Aug 17. Semin Immunol. 2006. PMID: 16914324 Review.
Cited by
-
Effective, safe, and sustained correction of murine XLA using a UCOE-BTK promoter-based lentiviral vector.Mol Ther Methods Clin Dev. 2021 Jan 20;20:635-651. doi: 10.1016/j.omtm.2021.01.007. eCollection 2021 Mar 12. Mol Ther Methods Clin Dev. 2021. PMID: 33718514 Free PMC article.
-
BAFF inhibition in SLE-Is tolerance restored?Immunol Rev. 2019 Nov;292(1):102-119. doi: 10.1111/imr.12810. Epub 2019 Sep 28. Immunol Rev. 2019. PMID: 31562657 Free PMC article. Review.
-
B Cells in Systemic Lupus Erythematosus: From Disease Mechanisms to Targeted Therapies.Rheum Dis Clin North Am. 2021 Aug;47(3):395-413. doi: 10.1016/j.rdc.2021.04.006. Epub 2021 Jun 16. Rheum Dis Clin North Am. 2021. PMID: 34215370 Free PMC article. Review.
-
B cell-activating factor (BAFF) from dendritic cells, monocytes and neutrophils is required for B cell maturation and autoantibody production in SLE-like autoimmune disease.Front Immunol. 2023 Feb 27;14:1050528. doi: 10.3389/fimmu.2023.1050528. eCollection 2023. Front Immunol. 2023. PMID: 36923413 Free PMC article.
-
TLR7 Signaling in Lupus B Cells: New Insights into Synergizing Factors and Downstream Signals.Curr Rheumatol Rep. 2021 Nov 24;23(11):80. doi: 10.1007/s11926-021-01047-1. Curr Rheumatol Rep. 2021. PMID: 34817709 Free PMC article. Review.
References
-
- Gavin AL, Duong B, Skog P, Aït-Azzouzene D, Greaves DR, Scott ML, and Nemazee D 2005. deltaBAFF, a splice isoform of BAFF, opposes full-length BAFF activity in vivo in transgenic mouse models. J. Immunol 175: 319–328. - PubMed
-
- Stohl W, Metyas S, Tan SM, Cheema GS, Oamar B, Xu D, Roschke V, Wu Y, Baker KP, and Hilbert DM 2003. B lymphocyte stimulator over-expression in patients with systemic lupus erythematosus: longitudinal observations. Arthritis Rheum 48: 3475–3486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

