AIF-1 and RNASET2 Play Complementary Roles in the Innate Immune Response of Medicinal Leech
- PMID: 30368505
- PMCID: PMC6738156
- DOI: 10.1159/000493804
AIF-1 and RNASET2 Play Complementary Roles in the Innate Immune Response of Medicinal Leech
Abstract
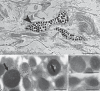
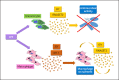
Recent studies demonstrated that allograft inflammatory factor-1 (AIF-1) and RNASET2 act as chemoattractants for macrophages and modulate the inflammatory processes in both vertebrates and invertebrates. The expression of these proteins significantly increases after bacterial infection; however, the mechanisms by which they regulate the innate immune response are still poorly defined. Here, we evaluate the effect of bacterial lipopolysaccharide injection on the expression pattern of these genes and the interrelation between them during innate immune response in the medicinal leech, an invertebrate model with a simple anatomy and a marked similarity with vertebrates in inflammatory processes. Collectively, prokaryotic-eukaryotic co-cultures and in vivo infection assays suggest that RNASET2 and AIF-1 play a crucial role in orchestrating a functional cross-talk between granulocytes and macrophages in leeches, resulting in the activation of an effective response against pathogen infection. RNASET2, firstly released by granulocytes, likely plays an early antibacterial role. Subsequently, AIF-1+ RNASET2-recruited macrophages further recruit other macrophages to potentiate the antibacterial inflammatory response. These experimental data are in keeping with the notion of RNA-SET2 acting as an alarmin-like molecule whose role is to locally transmit a "danger" signal (such as a bacterial infection) to the innate immune system in order to trigger an appropriate host response.
Keywords: Allograft inflammatory factor-1; Granulocytes; Leech innate immunity; Macrophages; RNASET2.
© 2018 The Author(s) Published by S. Karger AG, Basel.
Figures


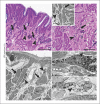
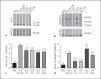
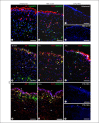
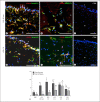

Similar articles
-
Antimicrobial Role of RNASET2 Protein During Innate Immune Response in the Medicinal Leech Hirudo verbana.Front Immunol. 2020 Mar 6;11:370. doi: 10.3389/fimmu.2020.00370. eCollection 2020. Front Immunol. 2020. PMID: 32210967 Free PMC article.
-
Human recombinant RNASET2-induced inflammatory response and connective tissue remodeling in the medicinal leech.Cell Tissue Res. 2017 May;368(2):337-351. doi: 10.1007/s00441-016-2557-9. Epub 2017 Jan 9. Cell Tissue Res. 2017. PMID: 28070637
-
Homolog of allograft inflammatory factor-1 induces macrophage migration during innate immune response in leech.Cell Tissue Res. 2015 Mar;359(3):853-64. doi: 10.1007/s00441-014-2058-7. Epub 2014 Dec 2. Cell Tissue Res. 2015. PMID: 25435328
-
Cumulative physiological events influence the inflammatory response of the bovine udder to Escherichia coli infections during the transition period.J Dairy Sci. 2007 Jun;90 Suppl 1:E39-54. doi: 10.3168/jds.2006-696. J Dairy Sci. 2007. PMID: 17517751 Review.
-
Allograft Inflammatory Factor-1 in Metazoans: Focus on Invertebrates.Biology (Basel). 2020 Oct 24;9(11):355. doi: 10.3390/biology9110355. Biology (Basel). 2020. PMID: 33114451 Free PMC article. Review.
Cited by
-
Diagnostic and therapeutic potential of RNASET2 in Crohn's disease: Disease-risk polymorphism modulates allelic-imbalance in expression and circulating protein levels and recombinant-RNASET2 attenuates pro-inflammatory cytokine secretion.Front Immunol. 2022 Nov 16;13:999155. doi: 10.3389/fimmu.2022.999155. eCollection 2022. Front Immunol. 2022. PMID: 36466822 Free PMC article.
-
HvRNASET2 Regulate Connective Tissue and Collagen I Remodeling During Wound Healing Process.Front Physiol. 2021 Feb 24;12:632506. doi: 10.3389/fphys.2021.632506. eCollection 2021. Front Physiol. 2021. PMID: 33716780 Free PMC article.
-
Searching for antimicrobial photosensitizers among a panel of BODIPYs.Photochem Photobiol Sci. 2022 Jul;21(7):1233-1248. doi: 10.1007/s43630-022-00212-4. Epub 2022 Apr 4. Photochem Photobiol Sci. 2022. PMID: 35377108
-
Regulation of neutrophil associated RNASET2 expression in rheumatoid arthritis.Sci Rep. 2024 Nov 5;14(1):26820. doi: 10.1038/s41598-024-77694-y. Sci Rep. 2024. PMID: 39500942 Free PMC article.
-
RNase T2 in Inflammation and Cancer: Immunological and Biological Views.Front Immunol. 2020 Aug 13;11:1554. doi: 10.3389/fimmu.2020.01554. eCollection 2020. Front Immunol. 2020. PMID: 32903619 Free PMC article. Review.
References
-
- Frantz S, Vincent KA, Feron O, Kelly RA. Innate immunity and angiogenesis. Circ Res. 2005 Jan;96((1)):15–26. - PubMed
-
- Baranzini N, Pedrini E, Girardello R, Tettamanti G, de Eguileor M, Taramelli R, et al. Human recombinant RNASET2-induced inflammatory response and connective tissue remodeling in the medicinal leech. Cell Tissue Res. 2017 May;368((2)):337–51. - PubMed
-
- Schorn T, Drago F, Tettamanti G, Valvassori R, de Eguileor M, Vizioli J, et al. Homolog of allograft inflammatory factor-1 induces macrophage migration during innate immune response in leech. Cell Tissue Res. 2015 Mar;359((3)):853–64. - PubMed
-
- Schorn T, Drago F, de Eguileor M, Valvassori R, Vizioli J, Tettamanti G, et al. The Allograft Inflammatory Factor-1 (AIF-1) homologous in Hirudo medicinalis (medicinal leech) is involved in immune response during wound healing and graft rejection processes. Invertebrate Surviv J. 2015;1:129–41.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

